mRNA export from mammalian cell nuclei is dependent on GANP
- PMID: 20005110
- PMCID: PMC2869303
- DOI: 10.1016/j.cub.2009.10.078
mRNA export from mammalian cell nuclei is dependent on GANP
Abstract
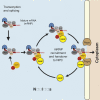
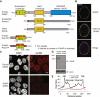
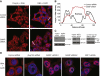
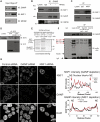
Bulk nuclear export of messenger ribonucleoproteins (mRNPs) through nuclear pore complexes (NPCs) is mediated by NXF1. It binds mRNPs through adaptor proteins such as ALY and SR splicing factors and mediates translocation through the central NPC transport channel via transient interactions with FG nucleoporins. Here, we show that mammalian cells require GANP (germinal center-associated nuclear protein) for efficient mRNP nuclear export and for efficient recruitment of NXF1 to NPCs. Separate regions of GANP show local homology to FG nucleoporins, the yeast mRNA export factor Sac3p, and the mammalian MCM3 acetyltransferase. GANP interacts with both NXF1 and NPCs and partitions between NPCs and the nuclear interior. GANP depletion inhibits mRNA export, with retention of mRNPs and NXF1 in punctate foci within the nucleus. The GANP N-terminal region that contains FG motifs interacts with the NXF1 FG-binding domain. Overexpression of this GANP fragment leads to nuclear accumulation of both poly(A)(+)RNA and NXF1. Treatment with transcription inhibitors redistributes GANP from NPCs into foci throughout the nucleus. These results establish GANP as an integral component of the mammalian mRNA export machinery and suggest a model whereby GANP facilitates the transfer of NXF1-containing mRNPs to NPCs.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
GANP enhances the efficiency of mRNA nuclear export in mammalian cells.Nucleus. 2010 Sep-Oct;1(5):393-6. doi: 10.4161/nucl.1.5.12351. Nucleus. 2010. PMID: 21326821 Free PMC article.
-
Functional and structural characterization of the mammalian TREX-2 complex that links transcription with nuclear messenger RNA export.Nucleic Acids Res. 2012 May;40(10):4562-73. doi: 10.1093/nar/gks059. Epub 2012 Feb 4. Nucleic Acids Res. 2012. PMID: 22307388 Free PMC article.
-
Selective nuclear export of specific classes of mRNA from mammalian nuclei is promoted by GANP.Nucleic Acids Res. 2014 Apr;42(8):5059-71. doi: 10.1093/nar/gku095. Epub 2014 Feb 7. Nucleic Acids Res. 2014. PMID: 24510098 Free PMC article.
-
Nuclear export of mRNA: from the site of transcription to the cytoplasm.Exp Cell Res. 2004 May 15;296(1):12-20. doi: 10.1016/j.yexcr.2004.03.015. Exp Cell Res. 2004. PMID: 15120988 Review.
-
The critical role of germinal center-associated nuclear protein in cell biology, immunohematology, and hematolymphoid oncogenesis.Exp Hematol. 2020 Oct;90:30-38. doi: 10.1016/j.exphem.2020.08.007. Epub 2020 Aug 20. Exp Hematol. 2020. PMID: 32827560 Review.
Cited by
-
GANP enhances the efficiency of mRNA nuclear export in mammalian cells.Nucleus. 2010 Sep-Oct;1(5):393-6. doi: 10.4161/nucl.1.5.12351. Nucleus. 2010. PMID: 21326821 Free PMC article.
-
MCM3AP is transcribed from a promoter within an intron of the overlapping gene for GANP.J Mol Biol. 2011 Feb 25;406(3):355-61. doi: 10.1016/j.jmb.2010.12.035. Epub 2010 Dec 30. J Mol Biol. 2011. PMID: 21195085 Free PMC article.
-
Structural basis for the assembly and nucleic acid binding of the TREX-2 transcription-export complex.Nat Struct Mol Biol. 2012 Feb 19;19(3):328-36. doi: 10.1038/nsmb.2235. Nat Struct Mol Biol. 2012. PMID: 22343721 Free PMC article.
-
The dynamic pathway of nuclear RNA in eukaryotes.Nucleus. 2013 May-Jun;4(3):195-205. doi: 10.4161/nucl.24434. Epub 2013 Apr 11. Nucleus. 2013. PMID: 23580182 Free PMC article. Review.
-
Transcription and mRNA export machineries SAGA and TREX-2 maintain monoubiquitinated H2B balance required for DNA repair.J Cell Biol. 2018 Oct 1;217(10):3382-3397. doi: 10.1083/jcb.201803074. Epub 2018 Jul 27. J Cell Biol. 2018. PMID: 30054449 Free PMC article.
References
-
- Zhou Z., Luo M.J., Straesser K., Katahira J., Hurt E., Reed R. The protein Aly links pre-messenger-RNA splicing to nuclear export in metazoans. Nature. 2000;407:401–405. - PubMed
-
- Huang Y., Gattoni R., Stevenin J., Steitz J.A. SR splicing factors serve as adapter proteins for TAP-dependent mRNA export. Mol. Cell. 2003;11:837–843. - PubMed
-
- Grant R.P., Hurt E., Neuhaus D., Stewart M. Structure of the C-terminal FG-nucleoporin binding domain of Tap/NXF1. Nat. Struct. Biol. 2002;9:247–251. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

