(1)H MRS detection of glycine residue of reduced glutathione in vivo
- PMID: 20005139
- PMCID: PMC2818741
- DOI: 10.1016/j.jmr.2009.11.013
(1)H MRS detection of glycine residue of reduced glutathione in vivo
Abstract
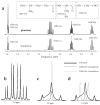
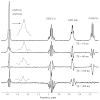
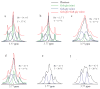
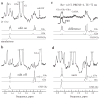
Glutathione (GSH) is a powerful antioxidant found inside different kinds of cells, including those of the central nervous system. Detection of GSH in the human brain using (1)H MR spectroscopy is hindered by low concentration and spectral overlap with other metabolites. Previous MRS methods focused mainly on the detection of the cysteine residue (GSH-Cys) via editing schemes. This study focuses on the detection of the glycine residue (GSH-Gly), which is overlapped by glutamate and glutamine (Glx) under physiological pH and temperature. The first goal of the study was to obtain the spectral parameters for characterization of the GSH-Gly signal under physiological conditions. The second goal was to investigate a new method of separating GSH-Gly from Glx in vivo. The characterization of the signal was carried out by utilization of numerical simulations as well as experiments over a wide range of magnetic fields (4.0-14T). The proposed separation scheme utilizes J-difference editing to quantify the Glx contribution to separate it from the GSH-Gly signal. The presented method retains 100% of the GSH-Gly signal. The overall increase in signal to noise ratio of the targeted resonance is calculated to yield a significant SNR improvement compared to previously used methods that target GSH-Cys residue. This allows shorter acquisition times for in vivo human clinical studies.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Quantification of glutathione transverse relaxation time T2 using echo time extension with variable refocusing selectivity and symmetry in the human brain at 7 Tesla.J Magn Reson. 2018 May;290:1-11. doi: 10.1016/j.jmr.2018.02.017. Epub 2018 Mar 1. J Magn Reson. 2018. PMID: 29524756
-
Intramolecular hydrogen transfer reactions of thiyl radicals from glutathione: formation of carbon-centered radical at Glu, Cys, and Gly.Chem Res Toxicol. 2012 Sep 17;25(9):1842-61. doi: 10.1021/tx3000494. Epub 2012 Jul 3. Chem Res Toxicol. 2012. PMID: 22712461 Free PMC article.
-
Simultaneous quantification of GABA, Glx and GSH in the neonatal human brain using magnetic resonance spectroscopy.Neuroimage. 2021 Jun;233:117930. doi: 10.1016/j.neuroimage.2021.117930. Epub 2021 Mar 9. Neuroimage. 2021. PMID: 33711485 Free PMC article.
-
Glutathione Conformations and Its Implications for in vivo Magnetic Resonance Spectroscopy.J Alzheimers Dis. 2017;59(2):537-541. doi: 10.3233/JAD-170350. J Alzheimers Dis. 2017. PMID: 28527221 Free PMC article. Review.
-
Precision of metabolite-selective MRS measurements of glutamate, GABA and glutathione: A review of human brain studies.NMR Biomed. 2024 Mar;37(3):e5071. doi: 10.1002/nbm.5071. Epub 2023 Dec 4. NMR Biomed. 2024. PMID: 38050448 Review.
Cited by
-
Quantification of glutathione in the human brain by MR spectroscopy at 3 Tesla: Comparison of PRESS and MEGA-PRESS.Magn Reson Med. 2017 Oct;78(4):1257-1266. doi: 10.1002/mrm.26532. Epub 2016 Oct 31. Magn Reson Med. 2017. PMID: 27797108 Free PMC article.
-
Edited 1 H magnetic resonance spectroscopy in vivo: Methods and metabolites.Magn Reson Med. 2017 Apr;77(4):1377-1389. doi: 10.1002/mrm.26619. Epub 2017 Feb 2. Magn Reson Med. 2017. PMID: 28150876 Free PMC article. Review.
-
Transverse relaxation time constants of the five major metabolites in human brain measured in vivo using LASER and PRESS at 3 T.Magn Reson Med. 2018 Mar;79(3):1260-1265. doi: 10.1002/mrm.26826. Epub 2017 Jul 10. Magn Reson Med. 2018. PMID: 28691380 Free PMC article.
-
In vivo 1 H MRS detection of cystathionine in human brain tumors.Magn Reson Med. 2019 Oct;82(4):1259-1265. doi: 10.1002/mrm.27810. Epub 2019 May 26. Magn Reson Med. 2019. PMID: 31131476 Free PMC article.
-
Highly specific determination of IDH status using edited in vivo magnetic resonance spectroscopy.Neuro Oncol. 2018 Jun 18;20(7):907-916. doi: 10.1093/neuonc/nox214. Neuro Oncol. 2018. PMID: 29126125 Free PMC article.
References
-
- Dringen R, Hirrlinger J. Glutathione pathways in the brain. Biol Chem. 2003;384:505–516. - PubMed
-
- Cruz R, Almaguer Melian W, Bergado Rosado JA. Glutathione in cognitive function and neurodegeneration. Rev Neurol. 2003;36:877–886. - PubMed
-
- Schulz J, Lindenau J, Seyfried J, Dichgans J. Glutathione, oxidative stress and neurodegeneration. Eur J Biochem. 2000;267:4904–4911. - PubMed
-
- Brown FF, Campbell ID, Kuchel PW, Rabenstein DC. Human erythrocyte metabolism studies by 1H spin echo NMR. FEBS Lett. 1977;82:12–16. - PubMed
-
- Rabenstein DL, Brown DW, McNeil CJ. Determination of glutathione in intact and hemolyzed erythrocytes by titration with tert-butyl hydroperoxide with end point detection by 1H nuclear magnetic resonance spectrometry. Anal Chem. 1985;57:2294–2299. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

