Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial
- PMID: 20005174
- PMCID: PMC3058239
- DOI: 10.1016/S1470-2045(09)70314-6
Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial
Abstract
Background: The 21-gene recurrence score assay is prognostic for women with node-negative, oestrogen-receptor-positive breast cancer treated with tamoxifen. A low recurrence score predicts little benefit of chemotherapy. For node-positive breast cancer, we investigated whether the recurrence score was prognostic in women treated with tamoxifen alone and whether it identified those who might not benefit from anthracycline-based chemotherapy, despite higher risks of recurrence.
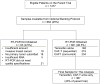
Methods: The phase 3 trial SWOG-8814 for postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer showed that chemotherapy with cyclophosphamide, doxorubicin, and fluorouracil (CAF) before tamoxifen (CAF-T) added survival benefit to treatment with tamoxifen alone. Optional tumour banking yielded specimens for determination of recurrence score by RT-PCR. In this retrospective analysis, we assessed the effect of recurrence score on disease-free survival by treatment group (tamoxifen vs CAF-T) using Cox regression, adjusting for number of positive nodes.
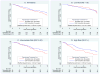
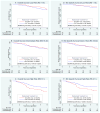
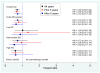
Findings: There were 367 specimens (40% of the 927 patients in the tamoxifen and CAF-T groups) with sufficient RNA for analysis (tamoxifen, n=148; CAF-T, n=219). The recurrence score was prognostic in the tamoxifen-alone group (p=0.006; hazard ratio [HR] 2.64, 95% CI 1.33-5.27, for a 50-point difference in recurrence score). There was no benefit of CAF in patients with a low recurrence score (score <18; log-rank p=0.97; HR 1.02, 0.54-1.93), but an improvement in disease-free survival for those with a high recurrence score (score > or =31; log-rank p=0.033; HR 0.59, 0.35-1.01), after adjustment for number of positive nodes. The recurrence score by treatment interaction was significant in the first 5 years (p=0.029), with no additional prediction beyond 5 years (p=0.58), although the cumulative benefit remained at 10 years. Results were similar for overall survival and breast-cancer-specific survival.
Interpretation: The recurrence score is prognostic for tamoxifen-treated patients with positive nodes and predicts significant benefit of CAF in tumours with a high recurrence score. A low recurrence score identifies women who might not benefit from anthracycline-based chemotherapy, despite positive nodes.
Funding: National Cancer Institute and Genomic Health.
Copyright (c) 2010 Elsevier Ltd. All rights reserved.
Figures


Comment in
-
First-generation genomic tests for breast cancer treatment.Lancet Oncol. 2010 Jan;11(1):6-7. doi: 10.1016/S1470-2045(09)70347-X. Epub 2009 Dec 10. Lancet Oncol. 2010. PMID: 20005177 No abstract available.
-
In the interest of full disclosure.Lancet Oncol. 2010 Apr;11(4):314-5; author reply 315. doi: 10.1016/S1470-2045(10)70044-9. Lancet Oncol. 2010. PMID: 20359661 No abstract available.
References
-
- Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL, Walker MG, Watson D, Park T, Hiller W, Fisher ER, Wickerham DL, Bryant J, Wolmark N. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–26. - PubMed
-
- Habel LA, Shak S, Jacobs MK, Capra A, Alexander C, Pho M, Baker J, Walker M, Watson D, Hackett J, Blick NT, Greenberg D, Fehrenbacher L, Langholz B, Quesenberry CP. A population-based study of tumor gene expression and risk of breast cancer death among lymph node-negative patients. Breast Cancer Research. 2006;8(3):R25. - PMC - PubMed
-
- Van de Vijver MJ, He YD, van’t Veer LJ, Dai H, Hart AAM, Voskuil DW, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;247:1999–2009. - PubMed
-
- Van’t Veer LJ, Paik S, Hayes DF. Gene expression profiling of breast cancer: a new tumor marker. J Clin Oncol. 2005;23(8):1631–5. - PubMed
-
- Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, Cronin W, Baehner BL, Watson D, Bryant J, Costantino J, Geyer CE, Jr, Wickerham DL, Wolmark N. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor–positive breast cancer. J Clin Oncol. 2006;24:3726–3734. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- N01 CA004919/CA/NCI NIH HHS/United States
- CA22433/CA/NCI NIH HHS/United States
- N01 CA046441/CA/NCI NIH HHS/United States
- U10 CA046368/CA/NCI NIH HHS/United States
- CA37981/CA/NCI NIH HHS/United States
- CA58348/CA/NCI NIH HHS/United States
- U10 CA022433/CA/NCI NIH HHS/United States
- CA58416/CA/NCI NIH HHS/United States
- U10 CA027057/CA/NCI NIH HHS/United States
- N01 CA035176/CA/NCI NIH HHS/United States
- CA76462/CA/NCI NIH HHS/United States
- CA45466/CA/NCI NIH HHS/United States
- U10 CA012644/CA/NCI NIH HHS/United States
- CA28862/CA/NCI NIH HHS/United States
- CA04920/CA/NCI NIH HHS/United States
- CA46136/CA/NCI NIH HHS/United States
- CA35261/CA/NCI NIH HHS/United States
- U10 CA037981/CA/NCI NIH HHS/United States
- N01 CA035431/CA/NCI NIH HHS/United States
- CA02599/CA/NCI NIH HHS/United States
- U10 CA016385/CA/NCI NIH HHS/United States
- CA35128/CA/NCI NIH HHS/United States
- U10 CA004919/CA/NCI NIH HHS/United States
- CA35117/CA/NCI NIH HHS/United States
- U10 CA046113/CA/NCI NIH HHS/United States
- CA12644/CA/NCI NIH HHS/United States
- U10 CA035128/CA/NCI NIH HHS/United States
- CA20319/CA/NCI NIH HHS/United States
- CA12213/CA/NCI NIH HHS/United States
- U10 CA045560/CA/NCI NIH HHS/United States
- CA58415/CA/NCI NIH HHS/United States
- U10 CA076447/CA/NCI NIH HHS/United States
- CA52650/CA/NCI NIH HHS/United States
- N01 CA032102/CA/NCI NIH HHS/United States
- CA35283/CA/NCI NIH HHS/United States
- N01 CA013612/CA/NCI NIH HHS/United States
- U10 CA035192/CA/NCI NIH HHS/United States
- U10 CA077202/CA/NCI NIH HHS/United States
- U10 CA021115/CA/NCI NIH HHS/United States
- U10 CA013612/CA/NCI NIH HHS/United States
- U10 CA058416/CA/NCI NIH HHS/United States
- CA35200/CA/NCI NIH HHS/United States
- CA58658/CA/NCI NIH HHS/United States
- CA35281/CA/NCI NIH HHS/United States
- CA35996/CA/NCI NIH HHS/United States
- N01 CA045807/CA/NCI NIH HHS/United States
- CA60138/CA/NCI NIH HHS/United States
- U10 CA014028/CA/NCI NIH HHS/United States
- N01 CA035119/CA/NCI NIH HHS/United States
- CA52757/CA/NCI NIH HHS/United States
- CA14028/CA/NCI NIH HHS/United States
- CA58882/CA/NCI NIH HHS/United States
- CA45377/CA/NCI NIH HHS/United States
- CA35084/CA/NCI NIH HHS/United States
- U10 CA035281/CA/NCI NIH HHS/United States
- CA58861/CA/NCI NIH HHS/United States
- CA52772/CA/NCI NIH HHS/United States
- CA35090/CA/NCI NIH HHS/United States
- CA46282/CA/NCI NIH HHS/United States
- CA76132/CA/NCI NIH HHS/United States
- N01 CA063844/CA/NCI NIH HHS/United States
- U10 CA035261/CA/NCI NIH HHS/United States
- U10 CA035178/CA/NCI NIH HHS/United States
- CA16385/CA/NCI NIH HHS/United States
- U10 CA045461/CA/NCI NIH HHS/United States
- CA76447/CA/NCI NIH HHS/United States
- U10 CA045450/CA/NCI NIH HHS/United States
- U10 CA032102/CA/NCI NIH HHS/United States
- U10 CA046282/CA/NCI NIH HHS/United States
- CA52623/CA/NCI NIH HHS/United States
- CA45450/CA/NCI NIH HHS/United States
- N01 CA035178/CA/NCI NIH HHS/United States
- CA77202-06/CA/NCI NIH HHS/United States
- CA46368/CA/NCI NIH HHS/United States
- N01 CA038926/CA/NCI NIH HHS/United States
- CA45461/CA/NCI NIH HHS/United States
- N01 CA027057/CA/NCI NIH HHS/United States
- U10 CA058686/CA/NCI NIH HHS/United States
- U10 CA046441/CA/NCI NIH HHS/United States
- U10 CA045377/CA/NCI NIH HHS/United States
- CA35192/CA/NCI NIH HHS/United States
- U10 CA058882/CA/NCI NIH HHS/United States
- U10 CA020319/CA/NCI NIH HHS/United States
- CA46113/CA/NCI NIH HHS/United States
- U10 CA038926/CA/NCI NIH HHS/United States
- CA32734/CA/NCI NIH HHS/United States
- CA58686/CA/NCI NIH HHS/United States
- U10 CA042777/CA/NCI NIH HHS/United States
- CA25224/CA/NCI NIH HHS/United States
- U10 CA035431/CA/NCI NIH HHS/United States
- CA67663,/CA/NCI NIH HHS/United States
- U10 CA035119/CA/NCI NIH HHS/United States
- CA42777/CA/NCI NIH HHS/United States
- CA52654/CA/NCI NIH HHS/United States
- U10 CA067663/CA/NCI NIH HHS/United States
- U10 CA052654/CA/NCI NIH HHS/United States
- U10 CA025224/CA/NCI NIH HHS/United States
- CA21115/CA/NCI NIH HHS/United States
- CA76429/CA/NCI NIH HHS/United States
- U10 CA035090/CA/NCI NIH HHS/United States
- U10 CA035176/CA/NCI NIH HHS/United States
- U10 CA058348/CA/NCI NIH HHS/United States
- CA58723/CA/NCI NIH HHS/United States
- U10 CA063844/CA/NCI NIH HHS/United States
- CA03096/CA/NCI NIH HHS/United States
- U10 CA058861/CA/NCI NIH HHS/United States
- U10 CA045807/CA/NCI NIH HHS/United States
- N01 CA045560/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

