A role of Histidine151 in the lamprey gonadotropin-releasing hormone receptor-1 (lGnRHR-1): Functional insight of diverse amino acid residues in the position of Tyr of the DRY motif in GnRHR from an ancestral type II receptor
- PMID: 20005226
- PMCID: PMC2856804
- DOI: 10.1016/j.ygcen.2009.12.001
A role of Histidine151 in the lamprey gonadotropin-releasing hormone receptor-1 (lGnRHR-1): Functional insight of diverse amino acid residues in the position of Tyr of the DRY motif in GnRHR from an ancestral type II receptor
Abstract
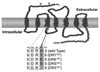
The highly conserved DRY motif located at the end of the third transmembrane of G-protein-coupled receptors has been described as a key motif for several aspects of GPCR functions. However, in the case of the vertebrate gonadotropin-releasing hormone receptor (GnRHR), the amino acid in the third position in the DRY motif is variable. In the lamprey, a most basal vertebrate, the third amino acid of the "DRY" in lamprey (lGnRHR-1) is His, while it is most often His/Gln in the type II GnRHR. To investigate the functional significance of the substitution of DRY to DRH in the GnRHR-1, second messenger signaling, ligand binding and internalization of the wild-type and mutant lGnRH receptors were characterized with site-directed mutagenesis. Treatment of the DRE(151) and DRS(151) mutant receptors with lamprey GnRH-I significantly reduced inositol phosphate compared to wild-type (DRH(151)) and DRY(151) receptors. The LogIC(50) of wild-type receptor (-9.554+/-0.049) was similar to the LogIC(50) of DRE(151), DRS(151) and DRX(151) mutants, yet these same mutants were shown to significantly reduce cell-surface expression. However, the DRY(151) mutant compared to the wild-type receptor increased cell-surface expression, suggesting that the reduction of IP production was due to the level of the cell-surface expression of the mutant receptors. The rate of internalization of DRX(151) (35.60%) was reduced compared to wild-type and other mutant receptors. These results suggest that His(151) of the lamprey GnRH receptor-1 may play a critical role in the retention of a certain level of cell-surface expression for subsequent cellular second messenger events.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures





References
-
- Alewijnse AE, Timmerman H, Jacobs EH, Smit MJ, Roovers E, Cotecchia S, Leurs R. The effect of mutations in the DRY motif on the constitutive activity and structural instability of the histamine H(2) receptor. Mol Pharmacol. 2000;57:890–898. - PubMed
-
- Arora KK, Cheng Z, Catt KJ. Mutations of the conserved DRS motif in the second intracellular loop of the gonadotropin-releasing hormone receptor affect expression, activation, and internalization. Mol Endocrinol. 1997;11:1203–1212. - PubMed
-
- Arora KK, Krsmanovic LZ, Mores N, O'Farrell H, Catt KJ. Mediation of cyclic AMP signaling by the first intracellular loop of the gonadotropin-releasing hormone receptor. J Biol Chem. 1998;273:25581–25586. - PubMed
-
- Arora KK, Sakai A, Catt KJ. Effects of second intracellular loop mutations on signal transduction and internalization of the gonadotropin-releasing hormone receptor. J Biol Chem. 1995;270:22820–22826. - PubMed
-
- Byrne B, McGregor A, Taylor PL, Sellar R, Rodger FE, Fraser HM, Eidne KA. Isolation and characterisation of the marmoset gonadotrophin releasing hormone receptor: Ser(140) of the DRS motif is substituted by Phe. J Endocrinol. 1999;163:447–456. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

