The electrostatics of VDAC: implications for selectivity and gating
- PMID: 20005234
- PMCID: PMC3736979
- DOI: 10.1016/j.jmb.2009.12.006
The electrostatics of VDAC: implications for selectivity and gating
Abstract
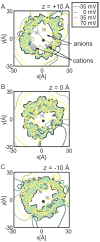
The voltage-dependent anion channel (VDAC) is the major pathway mediating the transfer of metabolites and ions across the mitochondrial outer membrane. Two hallmarks of the channel in the open state are high metabolite flux and anion selectivity, while the partially closed state blocks metabolites and is cation selective. Here we report the results from electrostatics calculations carried out on the recently determined high-resolution structure of murine VDAC1 (mVDAC1). Poisson-Boltzmann calculations show that the ion transfer free energy through the channel is favorable for anions, suggesting that mVDAC1 represents the open state. This claim is buttressed by Poisson-Nernst-Planck calculations that predict a high single-channel conductance indicative of the open state and an anion selectivity of 1.75--nearly a twofold selectivity for anions over cations. These calculations were repeated on mutant channels and gave selectivity changes in accord with experimental observations. We were then able to engineer an in silico mutant channel with three point mutations that converted mVDAC1 into a channel with a preference for cations. Finally, we investigated two proposals for how the channel gates between the open and the closed state. Both models involve the movement of the N-terminal helix, but neither motion produced the observed voltage sensitivity, nor did either model result in a cation-selective channel, which is observed experimentally. Thus, we were able to rule out certain models for channel gating, but the true motion has yet to be determined.
Copyright (c) 2009. Elsevier Ltd. All rights reserved.
Figures






References
-
- Schein SJ, Colombini M, Finkelstein A. Reconstitution in planar lipid bilayers of a voltage-dependent anion-selective channel obtained from paramecium mitochondria. J Membr Biol. 1976;30:99–120. - PubMed
-
- Colombini M. Voltage gating in the mitochondrial channel, VDAC. J Membr Biol. 1989;111:103–11. - PubMed
-
- Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–6. - PubMed
-
- Shimizu S, Narita M, Tsujimoto Y. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature. 1999;399:483–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

