Trafficking of G-protein-coupled receptors to the plasma membrane: insights for pharmacoperone drugs
- PMID: 20005736
- PMCID: PMC2831145
- DOI: 10.1016/j.tem.2009.11.003
Trafficking of G-protein-coupled receptors to the plasma membrane: insights for pharmacoperone drugs
Abstract
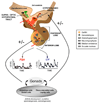
G protein-coupled receptors (GPCRs) are among the most common potential targets for pharmacological design. Synthesized in the endoplasmic reticulum, they interact with endogenous chaperones that assist in folding (or can retain incorrectly folded proteins) and are transferred to the plasma membrane where they exert their physiological functions. We summarize trafficking of the gonadotropin-releasing hormone receptor (GnRHR) to the plasma membrane. The trafficking of GnRHR is among the best characterized due in part to its small size and the consequent ease of making mutant proteins. Human mutations that cause disease through the misrouting of GPCRs including GnRHR are also reviewed. Special emphasis is placed on therapeutic opportunities presented by pharmacological chaperone drugs, or pharmacoperones, that allow misrouted mutants to be routed correctly and restored to function.
Copyright 2009 Elsevier Ltd. All rights reserved.
Figures
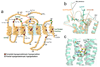

Similar articles
-
Use of pharmacoperones to reveal GPCR structural changes associated with constitutive activation and trafficking.Methods Enzymol. 2010;485:277-92. doi: 10.1016/B978-0-12-381296-4.00016-6. Methods Enzymol. 2010. PMID: 21050923
-
Therapeutic rescue of misfolded/mistrafficked mutants: automation-friendly high-throughput assays for identification of pharmacoperone drugs of GPCRs.Methods Enzymol. 2013;521:3-16. doi: 10.1016/B978-0-12-391862-8.00001-6. Methods Enzymol. 2013. PMID: 23351731
-
Restoration of testis function in hypogonadotropic hypogonadal mice harboring a misfolded GnRHR mutant by pharmacoperone drug therapy.Proc Natl Acad Sci U S A. 2013 Dec 24;110(52):21030-5. doi: 10.1073/pnas.1315194110. Epub 2013 Dec 9. Proc Natl Acad Sci U S A. 2013. PMID: 24324164 Free PMC article.
-
Pharmacological chaperones correct misfolded GPCRs and rescue function: protein trafficking as a therapeutic target.Subcell Biochem. 2012;63:263-89. doi: 10.1007/978-94-007-4765-4_14. Subcell Biochem. 2012. PMID: 23161143 Review.
-
Trafficking and quality control of the gonadotropin releasing hormone receptor in health and disease.Mol Cell Endocrinol. 2009 Feb 27;299(2):137-45. doi: 10.1016/j.mce.2008.10.051. Epub 2008 Nov 18. Mol Cell Endocrinol. 2009. PMID: 19059461 Free PMC article. Review.
Cited by
-
Targeting trafficking as a therapeutic avenue for misfolded GPCRs leading to endocrine diseases.Front Endocrinol (Lausanne). 2022 Aug 25;13:934685. doi: 10.3389/fendo.2022.934685. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36093106 Free PMC article. Review.
-
Functional rescue of Kallmann syndrome-associated prokineticin receptor 2 (PKR2) mutants deficient in trafficking.J Biol Chem. 2014 May 30;289(22):15518-26. doi: 10.1074/jbc.M114.556381. Epub 2014 Apr 21. J Biol Chem. 2014. PMID: 24753254 Free PMC article.
-
Ligands for Melanocortin Receptors: Beyond Melanocyte-Stimulating Hormones and Adrenocorticotropin.Biomolecules. 2022 Oct 1;12(10):1407. doi: 10.3390/biom12101407. Biomolecules. 2022. PMID: 36291616 Free PMC article. Review.
-
Misfolded G Protein-Coupled Receptors and Endocrine Disease. Molecular Mechanisms and Therapeutic Prospects.Int J Mol Sci. 2021 Nov 15;22(22):12329. doi: 10.3390/ijms222212329. Int J Mol Sci. 2021. PMID: 34830210 Free PMC article. Review.
-
A phenotypic high throughput screening assay for the identification of pharmacoperones for the gonadotropin releasing hormone receptor.Assay Drug Dev Technol. 2014 May;12(4):238-46. doi: 10.1089/adt.2014.576. Assay Drug Dev Technol. 2014. PMID: 24831790 Free PMC article.
References
-
- Lin JC, Liu HL. Protein conformational diseases: from mechanisms to drug designs. Curr Drug Discov Technol. 2006;3:145–153. - PubMed
-
- Castro-Fernandez C, et al. Beyond the signal sequence: protein routing in health and disease. Endocr Rev. 2005;26:479–503. - PubMed
-
- Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. - PubMed
-
- Gregersen N. Protein misfolding disorders: pathogenesis and intervention. J Inherit Metab Dis. 2006;29:456–470. - PubMed
-
- Beglova N, Blacklow SC. The LDL receptor: how acid pulls the trigger. Trends Biochem Sci. 2005;30:309–317. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

