A structure-based mechanism for vesicle capture by the multisubunit tethering complex Dsl1
- PMID: 20005805
- PMCID: PMC2806190
- DOI: 10.1016/j.cell.2009.11.002
A structure-based mechanism for vesicle capture by the multisubunit tethering complex Dsl1
Abstract
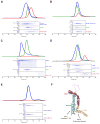
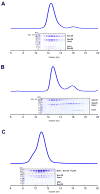
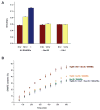
Vesicle trafficking requires membrane fusion, mediated by SNARE proteins, and upstream events that probably include "tethering," an initial long-range attachment between a vesicle and its target organelle. Among the factors proposed to mediate tethering are a set of multisubunit tethering complexes (MTCs). The Dsl1 complex, with only three subunits, is the simplest known MTC and is essential for the retrograde traffic of COPI-coated vesicles from the Golgi to the ER. To elucidate structural principles underlying MTC function, we have determined the structure of the Dsl1 complex, revealing a tower containing at its base the binding sites for two ER SNAREs and at its tip a flexible lasso for capturing vesicles. The Dsl1 complex binds to individual SNAREs via their N-terminal regulatory domains and also to assembled SNARE complexes; moreover, it is capable of accelerating SNARE complex assembly. Our results suggest that even the simplest MTC may be capable of orchestrating vesicle capture, uncoating, and fusion.
Figures
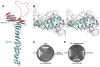

Comment in
-
A tethering complex recruits SNAREs and grabs vesicles.Cell. 2009 Dec 11;139(6):1053-5. doi: 10.1016/j.cell.2009.11.041. Cell. 2009. PMID: 20005800
References
-
- Adams PD, Grosse-Kunstleve RW, Hung LW, Ioerger TR, McCoy AJ, Moriarty NW, Read RJ, Sacchettini JC, Sauter NK, Terwilliger TC. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr D. 2002;58:1948–1954. - PubMed
-
- Andag U, Neumann T, Schmitt HD. The coatomer-interacting protein Dsl1p is required for Golgi-to-endoplasmic reticulum retrieval in yeast. J Biol Chem. 2001;276:39150–39160. - PubMed
-
- Andag U, Schmitt HD. Dsl1p, an essential component of the Golgi-endoplasmic reticulum retrieval system in yeast, uses the same sequence motif to interact with different subunits of the COPI vesicle coat. J Biol Chem. 2003;278:51722–51734. - PubMed
-
- Aoki T, Kojima M, Tani K, Tagaya M. Sec22b-dependent assembly of endoplasmic reticulum Q-SNARE proteins. Biochem J. 2008;410:93–100. - PubMed
-
- Bricogne G, Vonrhein C, Flensburg C, Schiltz M, Paciorek W. Generation, representation and flow of phase information in structure determination: recent developments in and around SHARP 2.0. Acta Crystallogr D. 2003;59:2023–2030. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

