Non-cell-autonomous stimulation of stem cell proliferation following ablation of Tcf3
- PMID: 20006604
- PMCID: PMC2834844
- DOI: 10.1016/j.yexcr.2009.12.005
Non-cell-autonomous stimulation of stem cell proliferation following ablation of Tcf3
Abstract
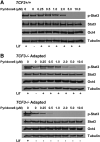
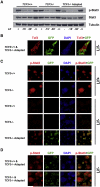
A combination of cell intrinsic factors and extracellular signals determine whether mouse embryonic stem cells (ESC) divide, self-renew, and differentiate. Here, we report a new interaction between cell intrinsic aspects of the canonical Wnt/Tcf/beta-catenin signaling pathway and extracellular Lif/Jak/Stat3 stimulation that combines to promote self-renewal and proliferation of ESC. Mutant ESC lacking the Tcf3 transcriptional repressor continue to self-renew in the absence of exogenous Lif and through pharmacological inhibition of Lif/Jak/Stat3 signaling; however, proliferation rates of TCF3-/- ESC were significantly decreased by inhibiting Jak/Stat3 activity. Cell mixing experiments showed that stimulation of Stat3 phosphorylation in TCF3-/- ESC was mediated through secretion of paracrine acting factors, but did not involve elevated Lif or LifR transcription. The new interaction between Wnt and Lif/Jak/Stat3 signaling pathways has potential for new insights into the growth of tumors caused by aberrant activity of Wnt/Tcf/beta-catenin signaling.
Copyright (c) 2009 Elsevier Inc. All rights reserved.
Figures
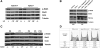


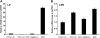

Similar articles
-
Opposing effects of Tcf3 and Tcf1 control Wnt stimulation of embryonic stem cell self-renewal.Nat Cell Biol. 2011 Jun 19;13(7):762-70. doi: 10.1038/ncb2283. Nat Cell Biol. 2011. PMID: 21685894 Free PMC article.
-
Human foreskin fibroblast produces interleukin-6 to support derivation and self-renewal of mouse embryonic stem cells.Stem Cell Res Ther. 2012 Jul 31;3(4):29. doi: 10.1186/scrt120. Stem Cell Res Ther. 2012. PMID: 22849865 Free PMC article.
-
Pramel7 mediates LIF/STAT3-dependent self-renewal in embryonic stem cells.Stem Cells. 2011 Mar;29(3):474-85. doi: 10.1002/stem.588. Stem Cells. 2011. PMID: 21425410
-
A WNTer revisit: new faces of β-catenin and TCFs in pluripotency.Sci Signal. 2011 Sep 27;4(193):pe41. doi: 10.1126/scisignal.2002436. Sci Signal. 2011. PMID: 21971038 Review.
-
[Signaling pathways regulating self-renewal of mouse embryonic stem cells--review].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2006 Dec;14(6):1248-52. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2006. PMID: 17204204 Review. Chinese.
Cited by
-
Intracellular Ca2+ Homeostasis and Nuclear Export Mediate Exit from Naive Pluripotency.Cell Stem Cell. 2019 Aug 1;25(2):210-224.e6. doi: 10.1016/j.stem.2019.04.015. Epub 2019 May 16. Cell Stem Cell. 2019. PMID: 31104942 Free PMC article.
-
Wnt pathway regulation of embryonic stem cell self-renewal.Cold Spring Harb Perspect Biol. 2012 Sep 1;4(9):a007971. doi: 10.1101/cshperspect.a007971. Cold Spring Harb Perspect Biol. 2012. PMID: 22952393 Free PMC article. Review.
-
Tcf7l1 directly regulates cardiomyocyte differentiation in embryonic stem cells.Stem Cell Res Ther. 2018 Oct 11;9(1):267. doi: 10.1186/s13287-018-1015-x. Stem Cell Res Ther. 2018. PMID: 30326964 Free PMC article.
-
Tcf7l1 proteins cell autonomously restrict cardiomyocyte and promote endothelial specification in zebrafish.Dev Biol. 2013 Aug 15;380(2):199-210. doi: 10.1016/j.ydbio.2013.05.016. Epub 2013 May 21. Dev Biol. 2013. PMID: 23707897 Free PMC article.
-
Modeling the Role of Wnt Signaling in Human and Drosophila Stem Cells.Genes (Basel). 2018 Feb 16;9(2):101. doi: 10.3390/genes9020101. Genes (Basel). 2018. PMID: 29462894 Free PMC article.
References
-
- Nusse R. Wnt signaling and stem cell control. Cell Res. 2008;18:523–527. - PubMed
-
- Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destree O, Clevers H. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–399. - PubMed
-
- van de Wetering M, Cavallo R, Dooijes D, van Beest M, van Es J, Loureiro J, Ypma A, Hursh D, Jones T, Bejsovec A, Peifer M, Mortin M, Clevers H. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell. 1997;88:789–799. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

