Time-frequency dynamics of resting-state brain connectivity measured with fMRI
- PMID: 20006716
- PMCID: PMC2827259
- DOI: 10.1016/j.neuroimage.2009.12.011
Time-frequency dynamics of resting-state brain connectivity measured with fMRI
Abstract
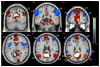
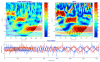
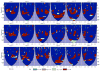
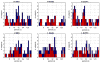
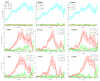
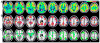
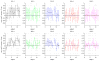
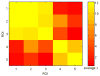
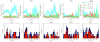
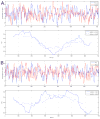
Most studies of resting-state functional connectivity using fMRI employ methods that assume temporal stationarity, such as correlation and data-driven decompositions computed across the duration of the scan. However, evidence from both task-based fMRI studies and animal electrophysiology suggests that functional connectivity may exhibit dynamic changes within time scales of seconds to minutes. In the present study, we investigated the dynamic behavior of resting-state connectivity across the course of a single scan, performing a time-frequency coherence analysis based on the wavelet transform. We focused on the connectivity of the posterior cingulate cortex (PCC), a primary node of the default-mode network, examining its relationship with both the "anticorrelated" ("task-positive") network as well as other nodes of the default-mode network. It was observed that coherence and phase between the PCC and the anticorrelated network was variable in time and frequency, and statistical testing based on Monte Carlo simulations revealed the presence of significant scale-dependent temporal variability. In addition, a sliding-window correlation procedure identified other regions across the brain that exhibited variable connectivity with the PCC across the scan, which included areas previously implicated in attention and salience processing. Although it is unclear whether the observed coherence and phase variability can be attributed to residual noise or modulation of cognitive state, the present results illustrate that resting-state functional connectivity is not static, and it may therefore prove valuable to consider measures of variability, in addition to average quantities, when characterizing resting-state networks.
Copyright (c) 2009 Elsevier Inc. All rights reserved.
Figures




References
-
- Almeida M, Botelho LF, da Silva AM, Brett M, Poline JB, Anton J-L, Andrade A. Time-frequency analysis of brain connectivity with fMRI: A new method based on Wavelet Transform Coherence. Organization for Human Brain Mapping, 12th Annual Meeting; Chicago. 2007. Poster 250 M-PM.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

