The TMEM16 protein family: a new class of chloride channels?
- PMID: 20006941
- PMCID: PMC2793353
- DOI: 10.1016/j.bpj.2009.09.024
The TMEM16 protein family: a new class of chloride channels?
Abstract
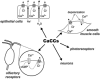
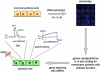
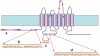
Cl(-) channels play important roles in many physiological processes, including transepithelial ion absorption and secretion, smooth and skeletal muscle contraction, neuronal excitability, sensory perception, and cell volume regulation. The molecular identity of many types of Cl(-) channels is still unknown. Recently, three research groups have arrived independently at the identification of TMEM16A (also known as anoctamin-1) as a membrane protein strongly related to the activity of Ca(2+)-activated Cl(-) channels (CaCCs). Site-specific mutagenesis of TMEM16A alters the properties of the channels, thus suggesting that TMEM16A forms, at least in part, the CaCC. TMEM16A is a member of a family that includes nine other membrane proteins. All TMEM16 proteins have a similar structure, with eight putative transmembrane domains and cytosolic amino- and carboxy-termini. TMEM16B expression also evokes the appearance of CaCCs, but with biophysical characteristics (voltage dependence, unitary conductance) different from those associated to TMEM16A. The roles of the other TMEM16 proteins are still unknown. The study of TMEM16 proteins may lead to identification of novel molecular mechanisms underlying ion transport and channel gating by voltage and Ca(2+).
Figures
References
-
- Jentsch T.J., Stein V., Weinreich F., Zdebik A.A. Molecular structure and physiological function of chloride channels. Physiol. Rev. 2002;82:503–568. - PubMed
-
- Hartzell C., Putzier I., Arreola J. Calcium-activated chloride channels. Annu. Rev. Physiol. 2005;67:719–758. - PubMed
-
- Frings S., Reuter D., Kleene S.J. Neuronal Ca2+-activated Cl− channels: homing in on an elusive channel species. Prog. Neurobiol. 2000;60:247–289. - PubMed
-
- Cunningham S.A., Awayda M.S., Bubien J.K., Ismailov I.I., Arrate M.P. Cloning of an epithelial chloride channel from bovine trachea. J. Biol. Chem. 1995;270:31016–31026. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

