A novel method to quantify contribution of channels and transporters to membrane potential dynamics
- PMID: 20006945
- PMCID: PMC2793359
- DOI: 10.1016/j.bpj.2009.08.060
A novel method to quantify contribution of channels and transporters to membrane potential dynamics
Abstract
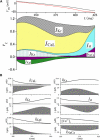
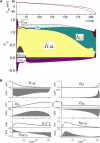
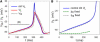
The action potential, once triggered in ventricular or atrial myocytes, automatically proceeds on its time course or is generated spontaneously in sinoatrial node pacemaker cells. It is induced by complex interactions among such cellular components as ion channels, transporters, intracellular ion concentrations, and signaling molecules. We have developed what is, to our knowledge, a new method using a mathematical model to quantify the contribution of each cellular component to the automatic time courses of the action potential. In this method, an equilibrium value, which the membrane potential is approaching at a given moment, is calculated along the time course of the membrane potential. The calculation itself is based on the time-varying conductance and the reversal potentials of individual ion channels and electrogenic ion transporters. Since the equilibrium potential moves in advance of the membrane potential change, we refer to it as the lead potential, V(L). The contribution of an individual current was successfully quantified by comparing dV(L)/dt before and after fixing the time-dependent change of a component of interest, such as the variations in the open probability of a channel or the turnover rate of an ion transporter. In addition to the action potential, the lead-potential analysis should also be applicable in all types of membrane excitation in many different kinds of cells.
Figures
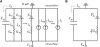


References
-
- Chay T.R., Lee Y.S. Studies on re-entrant arrhythmias and ectopic beats in excitable tissues by bifurcation analyses. J. Theor. Biol. 1992;155:137–171. - PubMed
-
- Kurata Y., Hisatome I., Imanishi S., Shibamoto T. Roles of L-type Ca2+ and delayed-rectifier K+ currents in sinoatrial node pacemaking: insights from stability and bifurcation analyses of a mathematical model. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H2804–H2819. - PubMed
-
- Kim T.H., Shin S.Y., Choo S.M., Cho K.H. Dynamical analysis of the calcium signaling pathway in cardiac myocytes based on logarithmic sensitivity analysis. Biotechnol. J. 2008;3:639–647. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

