Identification of two auto-cleavage products of nonstructural protein 1 (nsp1) in porcine reproductive and respiratory syndrome virus infected cells: nsp1 function as interferon antagonist
- PMID: 20006994
- PMCID: PMC7111964
- DOI: 10.1016/j.virol.2009.11.033
Identification of two auto-cleavage products of nonstructural protein 1 (nsp1) in porcine reproductive and respiratory syndrome virus infected cells: nsp1 function as interferon antagonist
Abstract
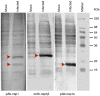
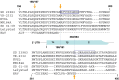
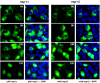
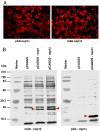
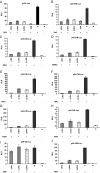
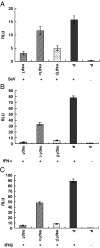
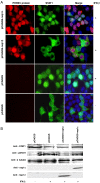
The porcine reproductive and respiratory syndrome virus nsp1 is predicted to be auto-cleaved from the replicase polyprotein into nsp1alpha and nsp1beta subunits. In infected cells, we detected the actual existence of nsp1alpha and nsp1beta. Cleavage sites between nsp1alpha/nsp1beta and nsp1beta/nsp2 were identified by protein microsequencing analysis. Time course study showed that nsp1alpha and nsp1beta mainly localize into the cell nucleus after 10 h post infection. Further analysis revealed that both proteins dramatically inhibited IFN-beta expression. The nsp1beta was observed to significantly inhibit expression from an interferon-stimulated response element promoter after Sendai virus infection or interferon treatment. It was further determined to inhibit nuclear translocation of STAT1 in the JAK-STAT signaling pathway. These results demonstrated that nsp1beta has ability to inhibit both interferon synthesis and signaling, while nsp1alpha alone strongly inhibits interferon synthesis. These findings provide important insights into mechanisms of nsp1 in PRRSV pathogenesis and its impact in vaccine development.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures
References
-
- Allende R., Lewis T.L., Lu Z., Rock D.L., Kutish G.F., Ali A., Doster A.R., Osorio F.A. North American and European porcine reproductive and respiratory syndrome viruses differ in non-structural protein coding regions. J. Gen. Virol. 1999;80:307–315. - PubMed
-
- Benfield D.A., Nelson E., Collins J.E., Harris L., Goyal S.M., Robison D., Christianson W.T., Morrison R.B., Gorcyca D., Chladek D. Characterization of swine infertility and respiratory syndrome (SIRS) virus (isolate ATCC VR-2332) J. Vet. Diagn. Invest. 1992;4:127–133. - PubMed
-
- Brown E., Lawson S., Welbon C., Murtaugh M.P., Nelson E.A., Zimmerman J.J., Rowland R.R.R., Fang Y. Antibody response of nonstructural proteins: implication for diagnostic detection and differentiation of Type I and Type II porcine reproductive and respiratory syndrome virus. Clin. Vaccine Immunol. 2009;16:628–635. - PMC - PubMed
-
- Buddaert W., Van Reeth K., Pensaert M. In vivo and in vitro interferon (IFN) studies with the porcine reproductive and respiratory syndrome virus (PRRSV) Adv. Exp. Med. Biol. 1998;440:461–467. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

