Acetazolamide and midazolam act synergistically to inhibit neuropathic pain
- PMID: 20007010
- PMCID: PMC2914558
- DOI: 10.1016/j.pain.2009.11.015
Acetazolamide and midazolam act synergistically to inhibit neuropathic pain
Abstract
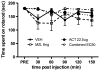
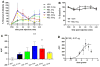
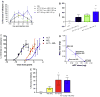
Treatment of neuropathic pain is a major clinical challenge that has been met with minimal success. After peripheral nerve injury, a decrease in the expression of the K-Cl cotransporter KCC2, a major neuronal Cl(-) extruder, leads to pathologic alterations in GABA(A) and glycine receptor function in the spinal cord. The down-regulation of KCC2 is expected to cause a reduction in Cl(-) extrusion capacity in dorsal horn neurons, which, together with the depolarizing efflux of HCO(3)(-) anions via GABA(A) channels, would result in a decrease in the efficacy of GABA(A)-mediated inhibition. Carbonic anhydrases (CA) facilitate intracellular HCO(3)(-) generation and hence, we hypothesized that inhibition of CAs would enhance the efficacy of GABAergic inhibition in the context of neuropathic pain. Despite the decrease in KCC2 expression, spinal administration of benzodiazepines has been shown to be anti-allodynic in neuropathic conditions. Thus, we also hypothesized that spinal inhibition of CAs might enhance the anti-allodynic effects of spinally administered benzodiazepines. Here, we show that inhibition of spinal CA activity with acetazolamide (ACT) reduces neuropathic allodynia. Moreover, we demonstrate that spinal co-administration of ACT and midazolam (MZL) act synergistically to reduce neuropathic allodynia after peripheral nerve injury. These findings indicate that the combined use of CA inhibitors and benzodiazepines may be effective in the clinical management of neuropathic pain in humans.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

References
-
- Benoliel R, Tal M, Eliav E. Effects of topiramate on the chronic constriction injury model in the rat. J Pain. 2006;7:878–83. - PubMed
-
- Blaesse P, Airaksinen MS, Rivera C, Kaila K. Cation-chloride cotransporters and neuronal function. Neuron. 2009;61:820–38. - PubMed
-
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

