Autocrine IGF-1/IGF-1R signaling is responsible for constitutive PI3K/Akt activation in acute myeloid leukemia: therapeutic value of neutralizing anti-IGF-1R antibody
- PMID: 20007139
- PMCID: PMC2833071
- DOI: 10.3324/haematol.2009.010785
Autocrine IGF-1/IGF-1R signaling is responsible for constitutive PI3K/Akt activation in acute myeloid leukemia: therapeutic value of neutralizing anti-IGF-1R antibody
Abstract
Background: Alterations in the PI3K/Akt pathway are found in a wide range of cancers and the development of PI3K inhibitors represents a promising approach to cancer therapy. Constitutive PI3K activation, reflecting an intrinsic oncogenic deregulation of primary blast cells, is detected in 50% of patients with acute myeloid leukemia. However, the mechanisms leading to this activation are currently unknown. As we previously reported IGF-1 autocriny in acute myeloid leukemia cells, we investigated whether IGF-1 signaling was involved in the constitutive activation of PI3K.
Design and methods: We analyzed the IGF-1/IGF-1R signaling pathway and PI3K activity in 40 acute myeloid leukemia bone marrow samples. Specific inhibition of IGF-1/IGF-1R signaling was investigated using neutralizing anti-IGF-1R, anti-IGF-1 antibodies or IGF-1 short interfering RNA. The anti-leukemic activity of the neutralizing anti-IGF-1R was tested by analyzing its effects on leukemic progenitor clonogenicity, blast cell proliferation and survival.
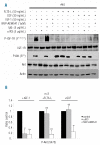
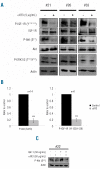
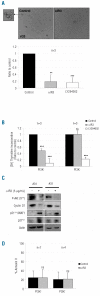
Results: In all samples tested, we found that functional IGF-1R was constantly expressed in leukemic cells. In the acute myeloid leukemia samples with PI3K activation, we found that the IGF-1R was constitutively phosphorylated, although no IGF-1R activating mutation was detected. Specific inhibition of IGF-1R signaling with neutralizing anti-IGF-1R strongly inhibited the constitutive phosphorylation of both IGF-1R and Akt in 70% of the PI3K activated samples. Moreover, both incubation with anti-IGF-1 antibody and IGF-1 short interfering RNA inhibited Akt phosphorylation in leukemic cells. Finally, neutralizing anti-IGF-1R treatment decreased the clonogenicity of leukemic progenitors and the proliferation of PI3K activated acute myeloid leukemia cells.
Conclusions: Our current data indicate a critical role for IGF-1 autocriny in constitutive PI3K/Akt activation in primary acute myeloid leukemia cells and provide a strong rationale for targeting IGF-1R as a potential new therapy for this disease.
Figures


References
-
- Appelbaum FR, Rowe JM, Radich J, Dick JE. Acute myeloid leukemia. Hematology Am Soc Hematol Educ Program. 2001:62–86. - PubMed
-
- Ravandi F, Burnett AK, Agura ED, Kantarjian HM. Progress in the treatment of acute myeloid leukemia. Cancer. 2007;110(9):1900–10. - PubMed
-
- Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299(5607):708–10. - PubMed
-
- Lee JW, Soung YH, Kim SY, Lee HW, Park WS, Nam SW, et al. PIK3CA gene is frequently mutated in breast carcinomas and hepatocellular carcinomas. Oncogene. 2005;24(8):1477–80. - PubMed
-
- Cairns P, Okami K, Halachmi S, Halachmi N, Esteller M, Herman JG, et al. Frequent inactivation of PTEN/MMAC1 in primary prostate cancer. Cancer Res. 1997;57(22):4997–5000. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

