Interleukin-3 promotes hemangioblast development in mouse aorta-gonad-mesonephros region
- PMID: 20007140
- PMCID: PMC2878783
- DOI: 10.3324/haematol.2009.014241
Interleukin-3 promotes hemangioblast development in mouse aorta-gonad-mesonephros region
Abstract
Background: The hemangioblast is a bi-potential precursor cell with the capacity to differentiate into hematopoietic and vascular cells. In mouse E7.0-7.5 embryos, the hemangioblast can be identified by a clonal blast colony-forming cell (BL-CFC) assay or single cell OP9 co-culture. However, the ontogeny of the hemangioblast in mid-gestation embryos is poorly defined.
Design and methods: The BL-CFC assay and the OP9 system were combined to illustrate the hemangioblast with lymphomyeloid and vascular potential in the mouse aorta-gonad-mesonephros region. The colony-forming assay, reverse transcriptase polymerase chain reaction analysis, immunostaining and flow cytometry were used to identify the hematopoietic potential, and Matrigel- or OP9-based methods were employed to evaluate endothelial progenitor activity.
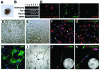
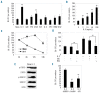
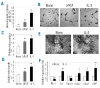
Results: Functionally, the aorta-gonad-mesonephros-derived BL-CFC produced erythroid/myeloid progenitors, CD19(+) B lymphocytes, and CD3(+)TCRbeta(+) T lymphocytes. Meanwhile, the BL-CFC-derived adherent cells generated CD31(+) tube-like structures on OP9 stromal cells, validating the endothelial progenitor potential. The aorta-gonad-mesonephros-derived hemangioblast was greatly enriched in CD31(+), endomucin(+) and CD105(+) subpopulations, which collectively pinpoints the endothelial layer as the main location. Interestingly, the BL-CFC was not detected in yolk sac, placenta, fetal liver or embryonic circulation. Screening of candidate cytokines revealed that interleukin-3 was remarkable in expanding the BL-CFC in a dose-dependent manner through the JAK2/STAT5 and MAPK/ERK pathways. Neutralizing interleukin-3 in the aorta-gonad-mesonephros region resulted in reduced numbers of BL-CFC, indicating the physiological requirement for this cytokine. Both hematopoietic and endothelial differentiation potential were significantly increased in interleukin-3-treated BL-CFC, suggesting a persistent positive influence. Intriguingly, interleukin-3 markedly amplified primitive erythroid and macrophage precursors in E7.5 embryos. Quantitative polymerase chain reaction analysis demonstrated declined Flk-1 and elevated Scl and von Willebrand factor transcription upon interleukin-3 stimulation, indicating accelerated hemangiopoiesis.
Conclusions: The hemangioblast with lymphomyeloid potential is one of the precursors of definitive hematopoiesis in the mouse aorta-gonad-mesonephros region. Interleukin-3 has a regulatory role with regards to both the number and capacity of the dual-potential hemangioblast.
Figures


Similar articles
-
Identification of high proliferative potential precursors with hemangioblastic activity in the mouse aorta-gonad- mesonephros region.Stem Cells. 2007 Jun;25(6):1423-30. doi: 10.1634/stemcells.2006-0556. Epub 2007 Mar 1. Stem Cells. 2007. PMID: 17332512
-
Characterization of hemangioblast in umbilical arteries of mid-gestation mouse embryos.Int J Hematol. 2012 Jun;95(6):632-9. doi: 10.1007/s12185-012-1068-z. Epub 2012 Apr 29. Int J Hematol. 2012. PMID: 22544769
-
Stimulation of mouse and human primitive hematopoiesis by murine embryonic aorta-gonad-mesonephros-derived stromal cell lines.Blood. 1998 Sep 15;92(6):2032-40. Blood. 1998. PMID: 9731061
-
Role of the microenvironment of the embryonic aorta-gonad-mesonephros region in hematopoiesis.Ann N Y Acad Sci. 2001 Jun;938:109-16. doi: 10.1111/j.1749-6632.2001.tb03579.x. Ann N Y Acad Sci. 2001. PMID: 11458497 Review.
-
[AGM region and hematopoiesis during ontogeny--review].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2005 Feb;13(1):164-8. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2005. PMID: 15748460 Review. Chinese.
Cited by
-
A 3D iPSC-differentiation model identifies interleukin-3 as a regulator of early human hematopoietic specification.Haematologica. 2021 May 1;106(5):1354-1367. doi: 10.3324/haematol.2019.228064. Haematologica. 2021. PMID: 32327499 Free PMC article.
-
Identification of the hemogenic endothelial progenitor and its direct precursor in human pluripotent stem cell differentiation cultures.Cell Rep. 2012 Sep 27;2(3):553-67. doi: 10.1016/j.celrep.2012.08.002. Epub 2012 Sep 13. Cell Rep. 2012. PMID: 22981233 Free PMC article.
-
Macrophages Derived From Human Induced Pluripotent Stem Cells: The Diversity of Protocols, Future Prospects, and Outstanding Questions.Front Cell Dev Biol. 2021 Jun 2;9:640703. doi: 10.3389/fcell.2021.640703. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34150747 Free PMC article. Review.
-
The inducible tissue-specific expression of the human IL-3/GM-CSF locus is controlled by a complex array of developmentally regulated enhancers.J Immunol. 2012 Nov 1;189(9):4459-69. doi: 10.4049/jimmunol.1201915. Epub 2012 Sep 28. J Immunol. 2012. PMID: 23024272 Free PMC article.
-
VEGF and FGF prime vascular tube morphogenesis and sprouting directed by hematopoietic stem cell cytokines.Blood. 2011 Apr 7;117(14):3709-19. doi: 10.1182/blood-2010-11-316752. Epub 2011 Jan 14. Blood. 2011. PMID: 21239704 Free PMC article.
References
-
- Ferkowicz MJ, Yoder MC. Blood island formation: longstanding observations and modern interpretations. Exp Hematol. 2005;33(9):1041–7. - PubMed
-
- Gekas C, Dieterlen-Lievre F, Orkin SH, Mikkola HK. The placenta is a niche for hematopoietic stem cells. Dev Cell. 2005;8(3):365–75. - PubMed
-
- McGrath KE, Palis J. Hematopoiesis in the yolk sac: more than meets the eye. Exp Hematol. 2005;33(9):1021–8. - PubMed
-
- Ottersbach K, Dzierzak E. The murine placenta contains hematopoietic stem cells within the vascular labyrinth region. Dev Cell. 2005;8(3):377–87. - PubMed
-
- Cumano A, Godin I. Ontogeny of the hematopoietic system. Annu Rev Immunol. 2007;25:745–85. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

