The fluid dynamics of canine olfaction: unique nasal airflow patterns as an explanation of macrosmia
- PMID: 20007171
- PMCID: PMC2871809
- DOI: 10.1098/rsif.2009.0490
The fluid dynamics of canine olfaction: unique nasal airflow patterns as an explanation of macrosmia
Abstract
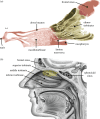
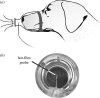
The canine nasal cavity contains hundreds of millions of sensory neurons, located in the olfactory epithelium that lines convoluted nasal turbinates recessed in the rear of the nose. Traditional explanations for canine olfactory acuity, which include large sensory organ size and receptor gene repertoire, overlook the fluid dynamics of odorant transport during sniffing. But odorant transport to the sensory part of the nose is the first critical step in olfaction. Here we report new experimental data on canine sniffing and demonstrate allometric scaling of sniff frequency, inspiratory airflow rate and tidal volume with body mass. Next, a computational fluid dynamics simulation of airflow in an anatomically accurate three-dimensional model of the canine nasal cavity, reconstructed from high-resolution magnetic resonance imaging scans, reveals that, during sniffing, spatially separate odour samples are acquired by each nostril that may be used for bilateral stimulus intensity comparison and odour source localization. Inside the nose, the computation shows that a unique nasal airflow pattern develops during sniffing, which is optimized for odorant transport to the olfactory part of the nose. These results contrast sharply with nasal airflow in the human. We propose that mammalian olfactory function and acuity may largely depend on odorant transport by nasal airflow patterns resulting from either the presence of a highly developed olfactory recess (in macrosmats such as the canine) or the lack of one (in microsmats including humans).
Figures

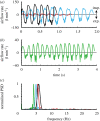

 , r2=0.92; (c)
, r2=0.92; (c)  , r2=0.93; (d) ∀insp.=2.15M0.99 ± 0.04, r2=0.87.
, r2=0.93; (d) ∀insp.=2.15M0.99 ± 0.04, r2=0.87.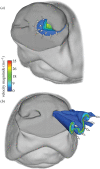

References
-
- Charles River Laboratories 2009. Long-Evans rats. See http://info.criver.com/flex_content_area/documents/rm_rm_c_long_evans_ra....
-
- Craven B. A. 2008. A fundamental study of the anatomy, aerodynamics, and transport phenomena of canine olfaction. University Park, PA: The Pennsylvania State University.
-
- Craven B. A., Neuberger T., Paterson E. G., Webb A. G., Josephson E. M., Morrison E. E., Settles G. S. 2007. Reconstruction and morphometric analysis of the nasal airway of the dog (Canis familiaris) and implications regarding olfactory airflow. Anat. Rec. 290, 1325–1340. ( 10.1002/ar.20592) - DOI - PubMed
-
- Craven B. A., Paterson E. G., Settles G. S. 2009. Development and verification of a high-fidelity computational fluid dynamics model of canine nasal airflow. J. Biomech. Eng. 131, 1–11. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
