Glucuronidation of dihydrotestosterone and trans-androsterone by recombinant UDP-glucuronosyltransferase (UGT) 1A4: evidence for multiple UGT1A4 aglycone binding sites
- PMID: 20007295
- PMCID: PMC2835394
- DOI: 10.1124/dmd.109.028712
Glucuronidation of dihydrotestosterone and trans-androsterone by recombinant UDP-glucuronosyltransferase (UGT) 1A4: evidence for multiple UGT1A4 aglycone binding sites
Abstract
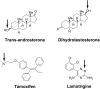
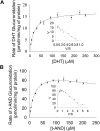
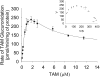
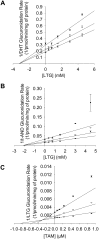
UDP-glucuronosyltransferase (UGT) 1A4-catalyzed glucuronidation is an important drug elimination pathway. Although atypical kinetic profiles (nonhyperbolic, non-Michaelis-Menten) of UGT1A4-catalyzed glucuronidation have been reported occasionally, systematic kinetic studies to explore the existence of multiple aglycone binding sites in UGT1A4 have not been conducted. To this end, two positional isomers, dihydrotestosterone (DHT) and trans-androsterone (t-AND), were used as probe substrates, and their glucuronidation kinetics with HEK293-expressed UGT1A4 were evaluated both alone and in the presence of a UGT1A4 substrate [tamoxifen (TAM) or lamotrigine (LTG)]. Coincubation with TAM, a high-affinity UGT1A4 substrate, resulted in a concentration-dependent activation/inhibition effect on DHT and t-AND glucuronidation, whereas LTG, a low-affinity UGT1A4 substrate, noncompetitively inhibited both processes. The glucuronidation kinetics of TAM were then evaluated both alone and in the presence of different concentrations of DHT or t-AND. TAM displayed substrate inhibition kinetics, suggesting that TAM may have two binding sites in UGT1A4. However, the substrate inhibition kinetic profile of TAM became more hyperbolic as the DHT or t-AND concentration was increased. Various two-site kinetic models adequately explained the interactions between TAM and DHT or TAM and t-AND. In addition, the effect of TAM on LTG glucuronidation was evaluated. In contrast to the mixed effect of TAM on DHT and t-AND glucuronidation, TAM inhibited LTG glucuronidation. Our results suggest that multiple aglycone binding sites exist within UGT1A4, which may result in atypical kinetics (both homotropic and heterotropic) in a substrate-dependent fashion.
Figures



References
-
- Chouinard S, Tessier M, Vernouillet G, Gauthier S, Labrie F, Barbier O, Bélanger A. (2006) Inactivation of the pure antiestrogen fulvestrant and other synthetic estrogen molecules by UDP-glucuronosyltransferase 1A enzymes expressed in breast tissue. Mol Pharmacol 69:908–920 - PubMed
-
- Fisher MB, Campanale K, Ackermann BL, VandenBranden M, Wrighton SA. (2000) In vitro glucuronidation using human liver microsomes and the pore-forming peptide alamethicin. Drug Metab Dispos 28:560–566 - PubMed
-
- Fujiwara R, Nakajima M, Yamanaka H, Katoh M, Yokoi T. (2008) Product inhibition of UDP-glucuronosyltransferase (UGT) enzymes by UDP obfuscates the inhibitory effects of UGT substrates. Drug Metab Dispos 36:361–367 - PubMed
-
- Galetin A, Clarke SE, Houston JB. (2002) Quinidine and haloperidol as modifiers of CYP3A4 activity: multisite kinetic model approach. Drug Metab Dispos 30:1512–1522 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

