Crystal structure of plant ferritin reveals a novel metal binding site that functions as a transit site for metal transfer in ferritin
- PMID: 20007325
- PMCID: PMC2823546
- DOI: 10.1074/jbc.M109.059790
Crystal structure of plant ferritin reveals a novel metal binding site that functions as a transit site for metal transfer in ferritin
Abstract
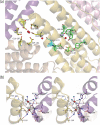
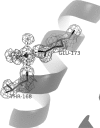
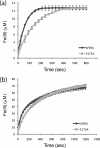
Ferritins are important iron storage and detoxification proteins that are widely distributed in living kingdoms. Because plant ferritin possesses both a ferroxidase site and a ferrihydrite nucleation site, it is a suitable model for studying the mechanism of iron storage in ferritin. This article presents for the first time the crystal structure of a plant ferritin from soybean at 1.8-A resolution. The soybean ferritin 4 (SFER4) had a high structural similarity to vertebrate ferritin, except for the N-terminal extension region, the C-terminal short helix E, and the end of the BC-loop. Similar to the crystal structures of other ferritins, metal binding sites were observed in the iron entry channel, ferroxidase center, and nucleation site of SFER4. In addition to these conventional sites, a novel metal binding site was discovered intermediate between the iron entry channel and the ferroxidase site. This site was coordinated by the acidic side chain of Glu(173) and carbonyl oxygen of Thr(168), which correspond, respectively, to Glu(140) and Thr(135) of human H chain ferritin according to their sequences. A comparison of the ferroxidase activities of the native and the E173A mutant of SFER4 clearly showed a delay in the iron oxidation rate of the mutant. This indicated that the glutamate residue functions as a transit site of iron from the 3-fold entry channel to the ferroxidase site, which may be universal among ferritins.
Figures



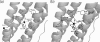

Similar articles
-
Dissecting the structural and functional roles of a putative metal entry site in encapsulated ferritins.J Biol Chem. 2020 Nov 13;295(46):15511-15526. doi: 10.1074/jbc.RA120.014502. Epub 2020 Sep 2. J Biol Chem. 2020. PMID: 32878987 Free PMC article.
-
The universal mechanism for iron translocation to the ferroxidase site in ferritin, which is mediated by the well conserved transit site.Biochem Biophys Res Commun. 2010 Sep 10;400(1):94-9. doi: 10.1016/j.bbrc.2010.08.017. Epub 2010 Aug 10. Biochem Biophys Res Commun. 2010. PMID: 20705053
-
Self-assembly is prerequisite for catalysis of Fe(II) oxidation by catalytically active subunits of ferritin.J Biol Chem. 2015 Oct 30;290(44):26801-10. doi: 10.1074/jbc.M115.678375. Epub 2015 Sep 14. J Biol Chem. 2015. PMID: 26370076 Free PMC article.
-
Mineralization in ferritin: an efficient means of iron storage.J Struct Biol. 1999 Jun 30;126(3):182-94. doi: 10.1006/jsbi.1999.4118. J Struct Biol. 1999. PMID: 10441528 Review.
-
Mechanisms of iron mineralization in ferritins: one size does not fit all.J Biol Inorg Chem. 2014 Aug;19(6):775-85. doi: 10.1007/s00775-014-1136-3. Epub 2014 Apr 19. J Biol Inorg Chem. 2014. PMID: 24748222 Review.
Cited by
-
Role of H-1 and H-2 subunits of soybean seed ferritin in oxidative deposition of iron in protein.J Biol Chem. 2010 Oct 15;285(42):32075-86. doi: 10.1074/jbc.M110.130435. Epub 2010 Aug 11. J Biol Chem. 2010. PMID: 20702403 Free PMC article.
-
Ferritin structure from Mycobacterium tuberculosis: comparative study with homologues identifies extended C-terminus involved in ferroxidase activity.PLoS One. 2011 Apr 8;6(4):e18570. doi: 10.1371/journal.pone.0018570. PLoS One. 2011. PMID: 21494619 Free PMC article.
-
Overexpression of wheat ferritin gene TaFER-5B enhances tolerance to heat stress and other abiotic stresses associated with the ROS scavenging.BMC Plant Biol. 2017 Jan 14;17(1):14. doi: 10.1186/s12870-016-0958-2. BMC Plant Biol. 2017. PMID: 28088182 Free PMC article.
-
H-Ferritin Is Preferentially Incorporated by Human Erythroid Cells through Transferrin Receptor 1 in a Threshold-Dependent Manner.PLoS One. 2015 Oct 6;10(10):e0139915. doi: 10.1371/journal.pone.0139915. eCollection 2015. PLoS One. 2015. PMID: 26441243 Free PMC article.
-
Moving metal ions through ferritin-protein nanocages from three-fold pores to catalytic sites.J Am Chem Soc. 2010 Oct 20;132(41):14562-9. doi: 10.1021/ja105583d. J Am Chem Soc. 2010. PMID: 20866049 Free PMC article.
References
-
- Harrison P. M., Arosio P. (1996) Biochim. Biophys. Acta 1275, 161–203 - PubMed
-
- Theil E. C. (1987) Annu. Rev. Biochem. 56, 289–315 - PubMed
-
- Arosio P., Ingrassia R., Cavadini P. (2009) Biochim. Biophys. Acta 1790, 589–599 - PubMed
-
- Lawson D. M., Artymiuk P. J., Yewdall S. J., Smith J. M., Livingstone J. C., Treffry A., Luzzago A., Levi S., Arosio P., Cesareni G., Thomas C. D., Shaw W. V., Harrison P. M. (1991) Nature 349, 541–544 - PubMed
-
- Hempstead P. D., Yewdall S. J., Fernie A. R., Lawson D. M., Artymiuk P. J., Rice D. W., Ford G. C., Harrison P. M. (1997) J. Mol. Biol. 268, 424–448 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

