The secret life of NAD+: an old metabolite controlling new metabolic signaling pathways
- PMID: 20007326
- PMCID: PMC2852209
- DOI: 10.1210/er.2009-0026
The secret life of NAD+: an old metabolite controlling new metabolic signaling pathways
Abstract
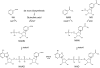
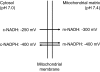
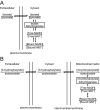
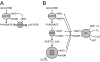
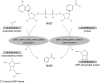
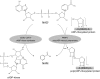
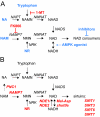
A century after the identification of a coenzymatic activity for NAD(+), NAD(+) metabolism has come into the spotlight again due to the potential therapeutic relevance of a set of enzymes whose activity is tightly regulated by the balance between the oxidized and reduced forms of this metabolite. In fact, the actions of NAD(+) have been extended from being an oxidoreductase cofactor for single enzymatic activities to acting as substrate for a wide range of proteins. These include NAD(+)-dependent protein deacetylases, poly(ADP-ribose) polymerases, and transcription factors that affect a large array of cellular functions. Through these effects, NAD(+) provides a direct link between the cellular redox status and the control of signaling and transcriptional events. Of particular interest within the metabolic/endocrine arena are the recent results, which indicate that the regulation of these NAD(+)-dependent pathways may have a major contribution to oxidative metabolism and life span extension. In this review, we will provide an integrated view on: 1) the pathways that control NAD(+) production and cycling, as well as its cellular compartmentalization; 2) the signaling and transcriptional pathways controlled by NAD(+); and 3) novel data that show how modulation of NAD(+)-producing and -consuming pathways have a major physiological impact and hold promise for the prevention and treatment of metabolic disease.
Figures
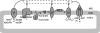
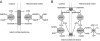
References
-
- Berger F, Ramírez-Hernández MH, Ziegler M 2004 The new life of a centenarian: signalling functions of NAD(P). Trends Biochem Sci 29:111–118 - PubMed
-
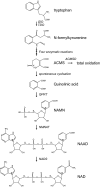
- Bender DA 1983 Biochemistry of tryptophan in health and disease. Mol Aspects Med 6:101–197 - PubMed
-
- Hegyi J, Schwartz RA, Hegyi V 2004 Pellagra: dermatitis, dementia, and diarrhea. Int J Dermatol 43:1–5 - PubMed
-
- Elvehjem C, Madden R, Strong F, Woolley D 1937 Relation of nicotinic acid and nicotinic acid amide to canine black tongue. J Am Chem Soc 59:1767–1768
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

