Using a functional enzyme model to understand the chemistry behind hydrogen sulfide induced hibernation
- PMID: 20007376
- PMCID: PMC2799705
- DOI: 10.1073/pnas.0904082106
Using a functional enzyme model to understand the chemistry behind hydrogen sulfide induced hibernation
Abstract
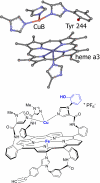
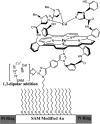
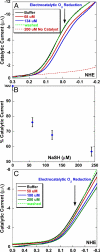
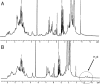
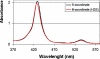
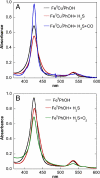
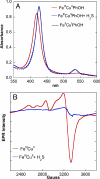
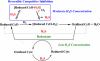
The toxic gas H(2)S is produced by enzymes in the body. At moderate concentrations, H(2)S elicits physiological effects similar to hibernation. Herein, we describe experiments that imply that the phenomenon probably results from reversible inhibition of the enzyme cytochrome c oxidase (CcO), which reduces oxygen during respiration. A functional model of the oxygen-reducing site in CcO was used to explore the effects of H(2)S during respiration. Spectroscopic analyses showed that the model binds two molecules of H2S. The electro-catalytic reduction of oxygen is reversibly inhibited by H(2)S concentrations similar to those that induce hibernation. This phenomenon derives from a weak, reversible binding of H(2)S to the Fe(II) porphyrin, which mimics heme a(3) in CcO's active site. No inhibition of CcO is detected at lower H(2)S concentrations. Nevertheless, at lower concentrations, H(2)S could have other biological effects on CcO. For example, H(2)S rapidly reduces Fe(III) and Cu(II) in both the oxidized form of this functional model and in CcO itself. H(2)S also reduces CcO's biological reductant, cytochrome c, which normally derives its reducing equivalents from food metabolism. Consequently, it is speculated that H(2)S might also serve as a source of electrons during periods of hibernation when food supplies are low.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Collman JP, Gagne RR, Reed CA. A paramagnetic dioxygen complex of iron II derived from a picket fence porphyrin further models for hemoproteins. J Am Chem Soc. 1974;96:2629–2631. - PubMed
-
- Collman JP, Gagne RR, Halbert TR, Marchon JC, Reed CA. Reversible oxygen adduct formation in ferrous complexes derived from a picket fence porphyrin model for oxy myoglobin. J Am Chem Soc. 1973;95:7868–7870. - PubMed
-
- Collman JP, et al. Picket-Fence Porphyrins. Synthetic Models for Oxygen Binding Hemoproteins. J Am Chem Soc. 1975;97:1427–1439. - PubMed
-
- Collman JP, Brauman JI, Suslick KS. Oxygen binding to iron porphyrins. J Am Chem Soc. 1975;97:7185–7186. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

