Selection and counterselection of the rtI233V adefovir resistance mutation during antiviral therapy
- PMID: 20007398
- PMCID: PMC2815635
- DOI: 10.1128/JCM.01073-09
Selection and counterselection of the rtI233V adefovir resistance mutation during antiviral therapy
Abstract
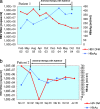
Recently, we reported on three patients with chronic hepatitis B virus (HBV) infection for whom adefovir (ADF) therapy virologically failed, most likely due to a preexisting rtI233V HBV polymerase mutation. Here, we describe two further patients with chronic HBV infection who were found to develop the rtI233V mutation after initiation of ADF therapy. These patients represent the first cases known so far in which the rtI233V ADF resistance mutation evolved under persistent HBV replication during HBV therapy with ADF. Interestingly, one of the previously described patients, who was initially successfully switched from ADF to tenofovir (TDF) and became virologically suppressed subsequently, experienced a moderate but remarkable rebound of HBV viremia after switching from TDF to entecavir, due to the emergence of renal toxicity. Thus, we provide evidence for the selection and counterselection of the rtI233V ADF resistance mutation during antiviral therapy.
Figures

Similar articles
-
Increased occurrence of mutant rtI233V of HBV in patients receiving adefovir therapy.Antivir Ther. 2016;21(1):9-16. doi: 10.3851/IMP2971. Epub 2015 Jun 16. Antivir Ther. 2016. PMID: 26079809
-
Variant of hepatitis B virus with primary resistance to adefovir.N Engl J Med. 2006 Apr 27;354(17):1807-12. doi: 10.1056/NEJMoa051214. N Engl J Med. 2006. PMID: 16641397
-
Hepatitis B virus wild-type and rtN236T populations show similar early HBV DNA decline in adefovir refractory patients on a tenofovir-based regimen.J Viral Hepat. 2013 Feb;20(2):131-40. doi: 10.1111/j.1365-2893.2012.01638.x. Epub 2012 Jul 9. J Viral Hepat. 2013. PMID: 23301548
-
[Therapy for chronic hepatitis B: relationship between HBV genotype and efficacy or resistance of anti-viral therapy].Nihon Shokakibyo Gakkai Zasshi. 2007 Oct;104(10):1459-64. Nihon Shokakibyo Gakkai Zasshi. 2007. PMID: 17917393 Review. Japanese. No abstract available.
-
[Resistance in hepatitis B virus].Enferm Infecc Microbiol Clin. 2008 May;26 Suppl 7:49-55. doi: 10.1016/s0213-005x(08)76519-4. Enferm Infecc Microbiol Clin. 2008. PMID: 19100231 Review. Spanish.
Cited by
-
The Molecular and Structural Basis of HBV-resistance to Nucleos(t)ide Analogs.J Clin Transl Hepatol. 2014 Sep;2(3):202-11. doi: 10.14218/JCTH.2014.00021. Epub 2014 Sep 15. J Clin Transl Hepatol. 2014. PMID: 26357626 Free PMC article. Review.
-
COLD-PCR Method for Early Detection of Antiviral Drug-Resistance Mutations in Treatment-Naive Children with Chronic Hepatitis B.Diagnostics (Basel). 2020 Jul 18;10(7):491. doi: 10.3390/diagnostics10070491. Diagnostics (Basel). 2020. PMID: 32708399 Free PMC article.
-
Clinical implications of hepatitis B virus mutations: recent advances.World J Gastroenterol. 2014 Jun 28;20(24):7653-64. doi: 10.3748/wjg.v20.i24.7653. World J Gastroenterol. 2014. PMID: 24976703 Free PMC article. Review.
-
Impact of rtI233V mutation in hepatitis B virus polymerase protein and adefovir efficacy: Homology modeling and molecular docking studies.Bioinformation. 2013;9(3):121-5. doi: 10.6026/97320630009121. Epub 2013 Feb 6. Bioinformation. 2013. PMID: 23423477 Free PMC article.
-
Comparison of the Mechanisms of Drug Resistance among HIV, Hepatitis B, and Hepatitis C.Viruses. 2010 Dec 1;2(12):2696-739. doi: 10.3390/v2122696. Viruses. 2010. PMID: 21243082 Free PMC article.
References
-
- Angus, P., R. Vaughan, S. Xiong, H. Yang, W. Delaney, C. Gibbs, C. Brosgart, D. Colledge, R. Edwards, A. Ayres, A. Bartholomeusz, and S. Locarnini. 2003. Resistance to adefovir dipivoxil therapy associated with the selection of a novel mutation in the HBV polymerase. Gastroenterology 125:292-297. - PubMed
-
- Bartholomeusz, A., M. Kuiper, P. Angus, A. Ayres, M. Littlejohn, D. Colledge, N. Warner, and S. Locarnini. 2006. Molecular analysis of HBV polymerase mutations associated with dual adefovir and lamivudine resistance. J. Hepatol. 44:S179-S179.
-
- Chang, T. T., and C. L. Lai. 2006. Hepatitis B virus with primary resistance to adefovir. N. Engl. J. Med. 355:322-323. - PubMed
-
- Curtis, M., Y. Zhu, and K. Borroto-Esoda. 2007. Hepatitis B virus containing the I233V mutation in the polymerase reverse-transcriptase domain remains sensitive to inhibition by adefovir. J. Infect. Dis. 196:1483-1486. - PubMed
-
- Degertekin, B., and A. S. Lok. 2009. Indications for therapy in hepatitis B. Hepatology 49:S129-S137. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

