Development and characterization of a three-dimensional organotypic human vaginal epithelial cell model
- PMID: 20007410
- PMCID: PMC6366157
- DOI: 10.1095/biolreprod.109.080408
Development and characterization of a three-dimensional organotypic human vaginal epithelial cell model
Abstract
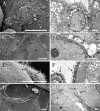
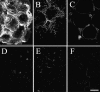
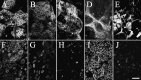
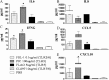
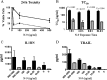
We have developed an in vitro human vaginal epithelial cell (EC) model using the innovative rotating wall vessel (RWV) bioreactor technology that recapitulates in vivo structural and functional properties, including a stratified squamous epithelium with microvilli, tight junctions, microfolds, and mucus. This three-dimensional (3-D) vaginal model provides a platform for high-throughput toxicity testing of candidate microbicides targeted to combat sexually transmitted infections, effectively complementing and extending existing testing systems such as surgical explants or animal models. Vaginal ECs were grown on porous, collagen-coated microcarrier beads in a rotating, low fluid-shear environment; use of RWV bioreactor technology generated 3-D vaginal EC aggregates. Immunofluorescence and scanning and transmission electron microscopy confirmed differentiation and polarization of the 3-D EC aggregates among multiple cell layers and identified ultrastructural features important for nutrient absorption, cell-cell interactions, and pathogen defense. After treatment with a variety of toll-like receptor (TLR) agonists, cytokine production was quantified by cytometric bead array, confirming that TLRs 2, 3, 5, and 6 were expressed and functional. The 3-D vaginal aggregates were more resistant to nonoxynol-9 (N-9), a contraceptive and previous microbicide candidate, when compared to two-dimensional monolayers of the same cell line. A dose-dependent production of tumor necrosis factor-related apoptosis-inducing ligand and interleukin-1 receptor antagonist, biomarkers of cervicovaginal inflammation, correlated to microbicide toxicity in the 3-D model following N-9 treatment. These results indicate that this 3-D vaginal model could be used as a complementary tool for screening microbicide compounds for safety and efficacy, thus improving success in clinical trials.
Figures

References
-
- Herbst-Kralovetz MM, Quayle AJ, Ficarra M, Greene S, Rose WA II, Chesson R, Spagnuolo RA, Pyles RB. Quantification and comparison of toll-like receptor expression and responsiveness in primary and immortalized human female lower genital tract epithelia. Am J Reprod Immunol 2008; 59:212–224. - PubMed
-
- Quayle AJ. The innate and early immune response to pathogen challenge in the female genital tract and the pivotal role of epithelial cells. J Reprod Immunol 2002; 57:61–79. - PubMed
-
- Fichorova RN, Rheinwald JG, Anderson DJ. Generation of papillomavirus-immortalized cell lines from normal human ectocervical, endocervical, and vaginal epithelium that maintains expression of tissue-specific differentiation proteins. Biol Reprod 1997; 57:847–855. - PubMed
-
- DeSouza MM, Lagow E, Carson DD. Mucin functions and expression in mammalian reproductive tract tissues. Biochem Biophys Res Comm 1998; 247:1–6. - PubMed
-
- Fedele L, Bianchi S, Berlanda N, Fontana E, Raffaelli R, Bulfoni A, Braidotti P. Neovaginal mucosa after Vecchietti's laparoscopic operation for Rokitansky syndrome: structural and ultrastructural study. Am J Obstet Gynecol 2006; 195:56–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

