High concentrations of HGF inhibit skeletal muscle satellite cell proliferation in vitro by inducing expression of myostatin: a possible mechanism for reestablishing satellite cell quiescence in vivo
- PMID: 20007454
- PMCID: PMC2838568
- DOI: 10.1152/ajpcell.00449.2009
High concentrations of HGF inhibit skeletal muscle satellite cell proliferation in vitro by inducing expression of myostatin: a possible mechanism for reestablishing satellite cell quiescence in vivo
Abstract
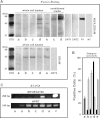
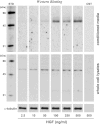
Skeletal muscle regeneration and work-induced hypertrophy rely on molecular events responsible for activation and quiescence of resident myogenic stem cells, satellite cells. Recent studies demonstrated that hepatocyte growth factor (HGF) triggers activation and entry into the cell cycle in response to mechanical perturbation, and that subsequent expression of myostatin may signal a return to cell quiescence. However, mechanisms responsible for coordinating expression of myostatin after an appropriate time lag following activation and proliferation are not clear. Here we address the possible role of HGF in quiescence through its concentration-dependent negative-feedback mechanism following satellite cell activation and proliferation. When activated/proliferating satellite cell cultures were treated for 24 h beginning 48-h postplating with 10-500 ng/ml HGF, the percentage of bromodeoxyuridine-incorporating cells decreased down to a baseline level comparable to 24-h control cultures in a HGF dose-dependent manner. The high level HGF treatment did not impair the cell viability and differentiation levels, and cells could be reactivated by lowering HGF concentrations to 2.5 ng/ml, a concentration that has been shown to optimally stimulate activation of satellite cells in culture. Coaddition of antimyostatin neutralizing antibody could prevent deactivation and abolish upregulation of cyclin-dependent kinase (Cdk) inhibitor p21. Myostatin mRNA expression was upregulated with high concentrations of HGF, as demonstrated by RT-PCR, and enhanced myostatin protein expression and secretion were revealed by Western blots of the cell lysates and conditioned media. These results indicate that HGF could induce satellite cell quiescence by stimulating myostatin expression. The HGF concentration required (over 10-50 ng/ml), however, is much higher than that for activation, which is initiated by rapid release of HGF from its extracellular association. Considering that HGF is produced by satellite cells and spleen and liver cells in response to muscle damage, local concentrations of HGF bathing satellite cells may reach a threshold sufficient to induce myostatin expression. This time lag may delay action of the quiescence signaling program in proliferating satellite cells during initial phases of muscle regeneration followed by induction of quiescence in a subset of cells during later phases.
Figures



Comment in
-
Dual effect of HGF on satellite/myogenic cell quiescence. Focus on "High concentrations of HGF inhibit skeletal muscle satellite cell proliferation in vitro by inducing expression of myostatin: a possible mechanism for reestablishing satellite cell quiescence in vivo".Am J Physiol Cell Physiol. 2010 Mar;298(3):C448-9. doi: 10.1152/ajpcell.00561.2009. Epub 2010 Jan 13. Am J Physiol Cell Physiol. 2010. PMID: 20071691 No abstract available.
References
-
- Allen RE, Boxhorn LK. Regulation of skeletal muscle satellite cell proliferation and differentiation by transforming growth factor-beta, insulin-like growth factor I, and fibroblast growth factor. J Cell Physiol 138: 311–315, 1989 - PubMed
-
- Allen RE, Sheehan SM, Taylor RG, Kendall TL, Rice GM. Hepatocyte growth factor activates quiescent skeletal muscle satellite cells in vitro. J Cell Physiol 165: 307–312, 1995 - PubMed
-
- Allen RE, Temm-Grove CJ, Sheehan SM, Rice GM. Skeletal muscle satellite cell cultures. Methods Cell Biol 52: 155–176, 1997 - PubMed
-
- Allen RE, Goll DE. Cellular and developmental biology of skeletal muscle as related to muscle growth. In: Biology of Growth of Domestic Animals, edited by Scanes CG. Ames, IA: Iowa State Press, 2003, p. 148–169
-
- Amthor H, Otto A, Macharia R, McKinnell I, Patel K. Myostatin imposes reversible quiescence on embryonic muscle precursors. Dev Dyn 235: 672–680, 2006 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

