The lysosomal sialic acid transporter sialin is required for normal CNS myelination
- PMID: 20007460
- PMCID: PMC2820501
- DOI: 10.1523/JNEUROSCI.3005-09.2009
The lysosomal sialic acid transporter sialin is required for normal CNS myelination
Abstract
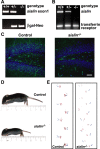
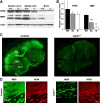
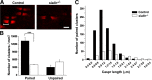
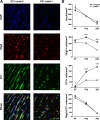
Salla disease and infantile sialic acid storage disease are autosomal recessive lysosomal storage disorders caused by mutations in the gene encoding sialin, a membrane protein that transports free sialic acid out of the lysosome after it is cleaved from sialoglycoconjugates undergoing degradation. Accumulation of sialic acid in lysosomes defines these disorders, and the clinical phenotype is characterized by neurodevelopmental defects, including severe CNS hypomyelination. In this study, we used a sialin-deficient mouse to address how loss of sialin leads to the defect in myelination. Behavioral analysis of the sialin(-/-) mouse demonstrates poor coordination, seizures, and premature death. Analysis by histology, electron microscopy, and Western blotting reveals a decrease in myelination of the CNS but normal neuronal cytoarchitecture and normal myelination of the PNS. To investigate potential mechanisms underlying CNS hypomyelination, we studied myelination and oligodendrocyte development in optic nerves. We found reduced numbers of myelinated axons in optic nerves from sialin(-/-) mice, but the myelin that was present appeared grossly normal. Migration and density of oligodendrocyte precursor cells were normal; however, a marked decrease in the number of postmitotic oligodendrocytes and an associated increase in the number of apoptotic cells during the later stages of myelinogenesis were observed. These findings suggest that a defect in maturation of cells in the oligodendrocyte lineage leads to increased apoptosis and underlies the myelination defect associated with sialin loss.
Figures



References
-
- Autio-Harmainen H, Oldfors A, Sourander P, Renlund M, Dammert K, Simila S. Neuropathology of Salla disease. Acta Neuropathol. 1988;75:481–490. - PubMed
-
- Barranger JA, Cabrera-Salazar MA. Lysosomal storage disorders. New York: Springer; 2007.
-
- Barres BA. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60:430–440. - PubMed
-
- Barres BA, Hart IK, Coles HS, Burne JF, Voyvodic JT, Richardson WD, Raff MC. Cell death and control of cell survival in the oligodendrocyte lineage. Cell. 1992;70:31–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
