cAMP-dependent axon guidance is distinctly regulated by Epac and protein kinase A
- PMID: 20007468
- PMCID: PMC6666109
- DOI: 10.1523/JNEUROSCI.3071-09.2009
cAMP-dependent axon guidance is distinctly regulated by Epac and protein kinase A
Abstract
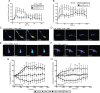
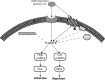
cAMP is a key mediator of a number of molecules that induce growth cone chemotaxis, including netrin-1 and myelin-associated glycoprotein (MAG). Endogenous neuronal cAMP levels decline during development, and concomitantly axonal growth cones switch their response to cAMP-dependent guidance cues from attraction to repulsion. The mechanisms by which cAMP regulates these polarized growth cone responses are unknown. We report that embryonic growth cone attraction to gradients of cAMP, netrin-1, or MAG is mediated by Epac. Conversely, the repulsion conferred by MAG or netrin-1 on adult growth cones is mediated by protein kinase A (PKA). Furthermore, fluorescence resonance energy transfer reveals that netrin-1 distinctly activates Epac in embryonic growth cones but PKA in postnatal neurons. Our results suggest that cAMP mediates growth cone attraction or repulsion by distinctly activating Epac or PKA, respectively. Moreover, we propose that the developmental switch in growth cone response to gradients of cAMP-dependent guidance cues from attraction to repulsion is the result of a switch from Epac- to PKA-mediated signaling pathways.
Figures


Similar articles
-
Protein kinase A regulates the sensitivity of spinal commissural axon turning to netrin-1 but does not switch between chemoattraction and chemorepulsion.J Neurosci. 2006 Mar 1;26(9):2419-23. doi: 10.1523/JNEUROSCI.5419-05.2006. J Neurosci. 2006. PMID: 16510719 Free PMC article.
-
Soluble adenylyl cyclase is not required for axon guidance to netrin-1.J Neurosci. 2008 Apr 9;28(15):3920-4. doi: 10.1523/JNEUROSCI.0547-08.2008. J Neurosci. 2008. PMID: 18400890 Free PMC article.
-
Electrical activity modulates growth cone guidance by diffusible factors.Neuron. 2001 Feb;29(2):441-52. doi: 10.1016/s0896-6273(01)00217-3. Neuron. 2001. PMID: 11239434
-
Axon guidance: growth cones make an unexpected turn.Curr Biol. 2002 Mar 19;12(6):R218-20. doi: 10.1016/s0960-9822(02)00755-8. Curr Biol. 2002. PMID: 11909551 Review.
-
[Netrin-1 and axonal guidance: signaling and asymmetrical translation].Med Sci (Paris). 2007 Mar;23(3):311-5. doi: 10.1051/medsci/2007233311. Med Sci (Paris). 2007. PMID: 17349294 Review. French.
Cited by
-
Divergent cAMP signaling differentially regulates serotonin-induced spinal motor plasticity.Neuropharmacology. 2017 Feb;113(Pt A):82-88. doi: 10.1016/j.neuropharm.2016.09.018. Epub 2016 Sep 20. Neuropharmacology. 2017. PMID: 27663700 Free PMC article.
-
Intracellular cAMP Sensor EPAC: Physiology, Pathophysiology, and Therapeutics Development.Physiol Rev. 2018 Apr 1;98(2):919-1053. doi: 10.1152/physrev.00025.2017. Physiol Rev. 2018. PMID: 29537337 Free PMC article. Review.
-
Rolipram-Loaded Polymeric Micelle Nanoparticle Reduces Secondary Injury after Rat Compression Spinal Cord Injury.J Neurotrauma. 2018 Feb 1;35(3):582-592. doi: 10.1089/neu.2017.5092. Epub 2018 Jan 3. J Neurotrauma. 2018. PMID: 29065765 Free PMC article.
-
Krüppel-Like Factors 9 and 13 Block Axon Growth by Transcriptional Repression of Key Components of the cAMP Signaling Pathway.Front Mol Neurosci. 2020 Nov 12;13:602638. doi: 10.3389/fnmol.2020.602638. eCollection 2020. Front Mol Neurosci. 2020. PMID: 33281552 Free PMC article.
-
Epac: new emerging cAMP-binding protein.BMB Rep. 2021 Mar;54(3):149-156. doi: 10.5483/BMBRep.2021.54.3.233. BMB Rep. 2021. PMID: 33298248 Free PMC article. Review.
References
-
- Bos JL. Epac: a new cAMP target and new avenues in cAMP research. Nat Rev Mol Cell Biol. 2003;4:733–738. - PubMed
-
- Christensen AE, Selheim F, de Rooij J, Dremier S, Schwede F, Dao KK, Martinez A, Maenhaut C, Bos JL, Genieser HG, Døskeland SO. cAMP analog mapping of Epac1 and cAMP kinase. Discriminating analogs demonstrate that Epac and cAMP kinase act synergistically to promote PC-12 cell neurite extension. J Biol Chem. 2003;278:35394–35402. - PubMed
-
- DeBellard ME, Tang S, Mukhopadhyay G, Shen YJ, Filbin MT. Myelin-associated glycoprotein inhibits axonal regeneration from a variety of neurons via interaction with sialoglycoprotein. Mol Cell Neurosci. 1996;7:89–101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
