No Nogo66- and NgR-mediated inhibition of regenerating axons in the zebrafish optic nerve
- PMID: 20007473
- PMCID: PMC6666099
- DOI: 10.1523/JNEUROSCI.3561-09.2009
No Nogo66- and NgR-mediated inhibition of regenerating axons in the zebrafish optic nerve
Abstract
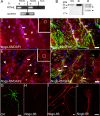
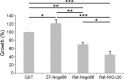
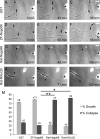
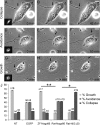
In contrast to mammals, lesioned axons in the zebrafish (ZF) optic nerve regenerate and restore vision. This correlates with the absence of the NogoA-specific N-terminal domains from the ZF nogo/rtn-4 (reticulon-4) gene that inhibits regeneration in mammals. However, mammalian nogo/rtn-4 carries a second inhibitory C-terminal domain, Nogo-66, being 70% identical with ZF-Nogo66. The present study examines, (1) whether ZF-Nogo66 is inhibitory and effecting similar signaling pathways upon Nogo66-binding to the Nogo66 receptor NgR and its coreceptors, and (2) whether Rat-Nogo66 on fish, and ZF-Nogo66 on mouse neurons, cause inhibition via NgR. Our results from "outgrowth, collapse and contact assays" suggest, surprisingly, that ZF-Nogo66 is growth-permissive for ZF and mouse neurons, quite in contrast to its Rat-Nogo66 homolog which inhibits growth. The opposite effects of ZF- and Rat-Nogo66 are, in both fish and mouse, transmitted by GPI (glycosylphosphatidylinositol)-anchored receptors, including NgR. The high degree of sequence homology in the predicted binding site is consistent with the ability of ZF- and mammalian-Nogo66 to bind to NgRs of both species. Yet, Rat-Nogo66 elicits phosphorylation of the downstream effector cofilin whereas ZF-Nogo66 has no influence on cofilin phosphorylation--probably because of significantly different Rat- versus ZF-Nogo66 sequences outside of the receptor-binding region effecting, by speculation, recruitment of a different set of coreceptors or microdomain association of NgR. Thus, not only was the NogoA-specific domain lost in fish, but Nogo66, the second inhibitory domain in mammals, and its signaling upon binding to NgR, was modified so that ZF-Nogo/RTN-4 does not impair axon regeneration.
Figures

References
-
- Ankerhold R, Stuermer CA. Fate of oligodendrocytes during retinal axon degeneration and regeneration in the goldfish visual pathway. J Neurobiol. 1999;41:572–584. - PubMed
-
- Atwal JA, Pinkston-Gosse, Syken J, Stawicki S, Wu Y, Shatz C, Tessier-Lavigne M. PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science. 2008;323:967–970. - PubMed
-
- Bastmeyer M, Bähr M, Stuermer CA. Fish optic nerve oligodendrocytes support axonal regeneration of fish and mammalian retinal ganglion cells. Glia. 1993;8:1–11. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
