Cellular mechanisms underlying burst firing in substantia nigra dopamine neurons
- PMID: 20007477
- PMCID: PMC2834564
- DOI: 10.1523/JNEUROSCI.2961-09.2009
Cellular mechanisms underlying burst firing in substantia nigra dopamine neurons
Abstract
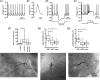
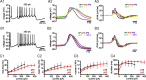
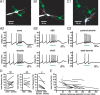
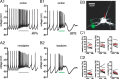
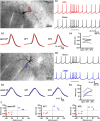
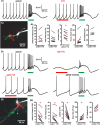
Burst firing of substantia nigra dopamine (SN DA) neurons is believed to represent an important teaching signal that instructs synaptic plasticity and associative learning. However, the mechanisms through which synaptic excitation overcomes the limiting effects of somatic Ca(2+)-dependent K(+) current to generate burst firing are controversial. Modeling studies suggest that synaptic excitation sufficiently amplifies oscillatory dendritic Ca(2+) and Na(+) channel currents to lead to the initiation of high-frequency firing in SN DA neuron dendrites. To test this model, visually guided compartment-specific patch-clamp recording and ion channel manipulation were applied to rodent SN DA neurons in vitro. As suggested previously, the axon of SN DA neurons was typically found to originate from a large-diameter dendrite that was proximal to the soma. However, in contrast to the predictions of the model, (1) somatic current injection generated firing that was similar in frequency and form to burst firing in vivo, (2) the efficacy of glutamatergic excitation was inversely related to the distance of excitation from the axon, (3) pharmacological blockade or genetic deletion of Ca(2+) channels did not prevent high-frequency firing, (4) action potential bursts were invariably detected first at sites that were proximal to the axon, and (5) pharmacological blockade of Na(+) channels in the vicinity of the axon/soma but not dendritic excitation impaired burst firing. Together, these data suggest that SN DA neurons integrate their synaptic input in a more conventional manner than was hypothesized previously.
Figures

References
-
- Becker C, Jick SS, Meier CR. Use of antihypertensives and the risk of Parkinson disease. Neurology. 2008;70:1438–1444. - PubMed
-
- Beckstead MJ, Grandy DK, Wickman K, Williams JT. Vesicular dopamine release elicits an inhibitory postsynaptic current in midbrain dopamine neurons. Neuron. 2004;42:939–946. - PubMed
-
- Blythe SN, Atherton JF, Bevan MD. Synaptic activation of dendritic AMPA and NMDA receptors generates transient high-frequency firing in substantia nigra dopamine neurons in vitro. J Neurophysiol. 2007;97:2837–2850. - PubMed
-
- Centonze D, Picconi B, Gubellini P, Bernardi G, Calabresi P. Dopaminergic control of synaptic plasticity in the dorsal striatum. Eur J Neurosci. 2001;13:1071–1077. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
