Altered sleep homeostasis after restraint stress in 5-HTT knock-out male mice: a role for hypocretins
- PMID: 20007481
- PMCID: PMC6666112
- DOI: 10.1523/JNEUROSCI.3138-09.2009
Altered sleep homeostasis after restraint stress in 5-HTT knock-out male mice: a role for hypocretins
Abstract
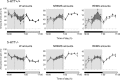
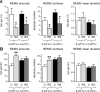
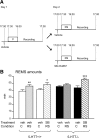
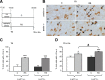
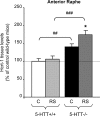
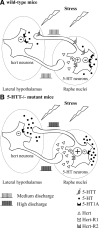
Restraint stress produces changes in the sleep pattern that are mainly characterized by a delayed increase in rapid eye movement sleep (REMS) amounts. Because the serotonin (5-HT) and the hypocretin (hcrt) systems that regulate REMS are interconnected, we used mutant mice deficient in the 5-HT transporter (5-HTT(-/-)) to examine the role of 5-HT and hcrt neurotransmissions in the sleep response to stress. In contrast to wild-type mice, restraint stress did not induce a delayed increase in REMS amounts in 5-HTT(-/-) mice, indicating impaired sleep homeostasis in mutants. However, pharmacological blockade of the hcrt type 1 receptor (hcrt-R1) before restraint stress restored the REMS increase in 5-HTT(-/-) mice. In line with this finding, 5-HTT(-/-) mutants displayed after restraint stress higher long-lasting activation of hypothalamic preprohcrt neurons than wild-type mice and elevated levels of the hcrt-1 peptide and the hcrt-R1 mRNA in the anterior raphe area. Thus, hypocretinergic neurotransmission was enhanced by stress in 5-HTT(-/-) mice. Furthermore, in 5-HTT(-/-) but not wild-type mice, hypothalamic levels of the 5-HT metabolite 5-hydroxyindole acetic acid significantly increased after restraint stress, indicating a marked enhancement of serotonergic neurotransmission in mutants. Altogether, our data show that increased serotonergic -and in turn hypocretinergic- neurotransmissions exert an inhibitory influence on stress-induced delayed REMS. We propose that the direct interactions between hcrt neurons in the hypothalamus and 5-HT neurons in the anterior raphe nuclei account, at least in part, for the adaptive sleep-wakefulness regulations triggered by acute stress.
Figures
Similar articles
-
Inactivation of median preoptic nucleus causes c-Fos expression in hypocretin- and serotonin-containing neurons in anesthetized rat.Brain Res. 2008 Oct 9;1234:66-77. doi: 10.1016/j.brainres.2008.07.115. Epub 2008 Aug 8. Brain Res. 2008. PMID: 18722360 Free PMC article.
-
Early life blockade of 5-hydroxytryptamine 1A receptors normalizes sleep and depression-like behavior in adult knock-out mice lacking the serotonin transporter.J Neurosci. 2006 May 17;26(20):5554-64. doi: 10.1523/JNEUROSCI.5156-05.2006. J Neurosci. 2006. PMID: 16707806 Free PMC article.
-
Neural substrates of awakening probed with optogenetic control of hypocretin neurons.Nature. 2007 Nov 15;450(7168):420-4. doi: 10.1038/nature06310. Epub 2007 Oct 17. Nature. 2007. PMID: 17943086 Free PMC article.
-
The role of dorsal raphe nucleus serotonergic and non-serotonergic neurons, and of their receptors, in regulating waking and rapid eye movement (REM) sleep.Sleep Med Rev. 2010 Oct;14(5):319-27. doi: 10.1016/j.smrv.2009.10.003. Epub 2010 Feb 12. Sleep Med Rev. 2010. PMID: 20153670 Review.
-
Serotonin control of sleep-wake behavior.Sleep Med Rev. 2011 Aug;15(4):269-81. doi: 10.1016/j.smrv.2010.11.003. Epub 2011 Apr 2. Sleep Med Rev. 2011. PMID: 21459634 Review.
Cited by
-
Chronic restraint stress impairs voluntary wheel running but has no effect on food-motivated behavior in mice.Brain Behav Immun. 2023 Jan;107:319-329. doi: 10.1016/j.bbi.2022.10.017. Epub 2022 Oct 29. Brain Behav Immun. 2023. PMID: 36349643 Free PMC article.
-
Effects of Social Defeat Stress on Sleep in Mice.Front Behav Neurosci. 2017 Nov 28;11:227. doi: 10.3389/fnbeh.2017.00227. eCollection 2017. Front Behav Neurosci. 2017. PMID: 29234278 Free PMC article.
-
Physiologically relevant changes in serotonin resolved by fast microdialysis.ACS Chem Neurosci. 2013 May 15;4(5):790-8. doi: 10.1021/cn400072f. Epub 2013 Apr 24. ACS Chem Neurosci. 2013. PMID: 23614776 Free PMC article.
-
A Bitter Experience That Enlightens the Future: COVID-19 Neurological Affection and Perspectives on the Orexigenic System.Cureus. 2022 Oct 28;14(10):e30788. doi: 10.7759/cureus.30788. eCollection 2022 Oct. Cureus. 2022. PMID: 36457603 Free PMC article. Review.
-
Hypocretinergic interactions with the serotonergic system regulate REM sleep and cataplexy.Nat Commun. 2020 Nov 27;11(1):6034. doi: 10.1038/s41467-020-19862-y. Nat Commun. 2020. PMID: 33247179 Free PMC article.
References
-
- Adamec R, Burton P, Blundell J, Murphy DL, Holmes A. Vulnerability to mild predator stress in serotonin transporter knockout mice. Behav Brain Res. 2006;170:126–140. - PubMed
-
- Adrien J, Alexandre C, Boutrel B, Popa D. Contribution of the “knock-out” technology to understanding the role of serotonin in sleep regulations. Arch Ital Biol. 2004;142:369–377. - PubMed
-
- Al-Barazanji KA, Wilson S, Baker J, Jessop DS, Harbuz MS. Central orexin-A activates hypothalamic-pituitary-adrenal axis and stimulates hypothalamic corticotropin releasing factor and arginine vasopressin neurones in conscious rats. J Neuroendocrinol. 2001;13:421–424. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
