Neuronal avalanches imply maximum dynamic range in cortical networks at criticality
- PMID: 20007483
- PMCID: PMC3862241
- DOI: 10.1523/JNEUROSCI.3864-09.2009
Neuronal avalanches imply maximum dynamic range in cortical networks at criticality
Abstract
Spontaneous neuronal activity is a ubiquitous feature of cortex. Its spatiotemporal organization reflects past input and modulates future network output. Here we study whether a particular type of spontaneous activity is generated by a network that is optimized for input processing. Neuronal avalanches are a type of spontaneous activity observed in superficial cortical layers in vitro and in vivo with statistical properties expected from a network operating at "criticality." Theory predicts that criticality and, therefore, neuronal avalanches are optimal for input processing, but until now, this has not been tested in experiments. Here, we use cortex slice cultures grown on planar microelectrode arrays to demonstrate that cortical networks that generate neuronal avalanches benefit from a maximized dynamic range, i.e., the ability to respond to the greatest range of stimuli. By changing the ratio of excitation and inhibition in the cultures, we derive a network tuning curve for stimulus processing as a function of distance from criticality in agreement with predictions from our simulations. Our findings suggest that in the cortex, (1) balanced excitation and inhibition establishes criticality, which maximizes the range of inputs that can be processed, and (2) spontaneous activity and input processing are unified in the context of critical phenomena.
Figures
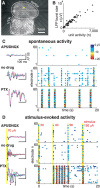
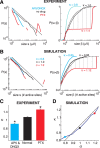
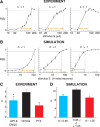

References
-
- Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science. 1996;273:1868–1871. - PubMed
-
- Bertschinger N, Natschläger T. Real-time computation at the edge of chaos in recurrent neural networks. Neural Comput. 2004;16:1413–1436. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
