Lack of host SPARC enhances vascular function and tumor spread in an orthotopic murine model of pancreatic carcinoma
- PMID: 20007485
- PMCID: PMC2806901
- DOI: 10.1242/dmm.003228
Lack of host SPARC enhances vascular function and tumor spread in an orthotopic murine model of pancreatic carcinoma
Abstract
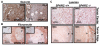
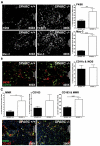
Utilizing subcutaneous tumor models, we previously validated SPARC (secreted protein acidic and rich in cysteine) as a key component of the stromal response, where it regulated tumor size, angiogenesis and extracellular matrix deposition. In the present study, we demonstrate that pancreatic tumors grown orthotopically in Sparc-null (Sparc(-/-)) mice are more metastatic than tumors grown in wild-type (Sparc(+/+)) littermates. Tumors grown in Sparc(-/-) mice display reduced deposition of fibrillar collagens I and III, basement membrane collagen IV and the collagen-associated proteoglycan decorin. In addition, microvessel density and pericyte recruitment are reduced in tumors grown in the absence of host SPARC. However, tumors from Sparc(-/-) mice display increased permeability and perfusion, and a subsequent decrease in hypoxia. Finally, we found that tumors grown in the absence of host SPARC exhibit an increase in alternatively activated macrophages. These results suggest that increased tumor burden in the absence of host SPARC is a consequence of reduced collagen deposition, a disrupted vascular basement membrane, enhanced vascular function and an immune-tolerant, pro-metastatic microenvironment.
Figures
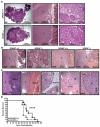
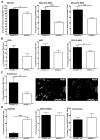

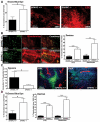
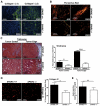

References
-
- Baluk P, Hashizume H, McDonald DM. (2005). Cellular abnormalities of blood vessels as targets in cancer. Curr Opin Genet Dev. 15, 102–111 - PubMed
-
- Bernstein EF, Fisher LW, Li K, LeBaron RG, Tan EM, Uitto J. (1995). Differential expression of the versican and decorin genes in photoaged and sun-protected skin. Comparison by immunohistochemical and northern analyses. Lab Invest. 72, 662–669 - PubMed
-
- Bernstein EF, Chen YQ, Kopp JB, Fisher L, Brown DB, Hahn PJ, Robey FA, Lakkakorpi J, Uitto J. (1996). Long-term sun exposure alters the collagen of the papillary dermis. Comparison of sun-protected and photoaged skin by northern analysis, immunohistochemical staining, and confocal laser scanning microscopy. J Am Acad Dermatol. 34, 209–218 - PubMed
-
- Bingle L, Brown NJ, Lewis CE. (2002). The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 196, 254–265 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

