Developmental alteration of endocannabinoid retrograde signaling in the hippocampus
- PMID: 20007500
- PMCID: PMC2822685
- DOI: 10.1152/jn.00327.2009
Developmental alteration of endocannabinoid retrograde signaling in the hippocampus
Abstract
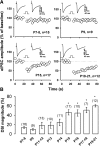
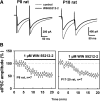
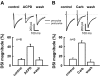
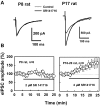
Endocannabinoids are lipid derivatives that mediate paracrine and juxtacrine signaling between cells. In the hippocampal CA1 region, a retrograde endocannabinoid signal suppresses GABA release by acting on presynaptic cannabinoid receptor-1 (CB1) and can be functionally manifested as depolarization-induced suppression of inhibition (DSI). In the present study, whole cell patch-clamp recordings in hippocampal slices were made to examine DSI in rats from P7-P21. Robust DSI develops in rat hippocampus at postnatal ages greater than two weeks, but only modest DSI is observed in P7-9 rat. DSI in neonatal rats can be enhanced by activation of group I metabotropic glutamate receptors (mGluRs) or muscarinic acetylcholine receptors in those neonatal rats. The DSI is also enhanced by sustained low-frequency (1 Hz) stimulation (5 min). This stimulus-enhanced DSI was prevented in the presence of 6-methyl-2-(phenylethynyl)-pyridine (10 microM), a group I mGluR antagonist. WIN55212-2, a synthetic CB1 agonist, produced a similar level of inhibition of GABAergic synaptic transmission at different postnatal time points. Therefore postsynaptic mechanisms appear to be mainly responsible for developmental changes in DSI, although presynaptic mechanisms cannot be ruled out entirely. We have also obtained evidence that tonic endocannabinoid release suppresses GABAergic transmission in the mature but not the neonatal hippocampus. The differential DSI magnitude at different stages of maturation could alter synaptic plasticity and learning and memory during hippocampal development.
Figures


References
-
- Bolshakov VY, Siegelbaum SA. Postsynaptic induction and presynaptic expression of hippocampal long-term depression. Science 264: 1148–1152, 1994 - PubMed
-
- Carlson G, Wang Y, Alger BE. Endocannabinoids facilitate the induction of LTP in the hippocampus. Nat Neurosci 5: 723–724, 2002 - PubMed
-
- Chevaleyre V, Castillo PE. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron 38: 461–72, 2003 - PubMed
-
- Collard KJ, Edwards R, Liu Y. Changes in synaptosomal glutamate release during postnatal development in the rat hippocampus and cortex. Brain Res 71: 37–43, 1993 - PubMed
-
- Defagot MC, Villar MJ, Antonelli MC. Differential localization of metabotropic glutamate receptors during postnatal development. Dev Neurosci 24: 272–282, 2002 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

