Merging of healthy motor modules predicts reduced locomotor performance and muscle coordination complexity post-stroke
- PMID: 20007501
- PMCID: PMC2822696
- DOI: 10.1152/jn.00825.2009
Merging of healthy motor modules predicts reduced locomotor performance and muscle coordination complexity post-stroke
Abstract
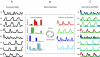
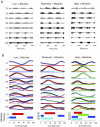
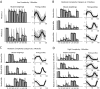
Evidence suggests that the nervous system controls motor tasks using a low-dimensional modular organization of muscle activation. However, it is not clear if such an organization applies to coordination of human walking, nor how nervous system injury may alter the organization of motor modules and their biomechanical outputs. We first tested the hypothesis that muscle activation patterns during walking are produced through the variable activation of a small set of motor modules. In 20 healthy control subjects, EMG signals from eight leg muscles were measured across a range of walking speeds. Four motor modules identified through nonnegative matrix factorization were sufficient to account for variability of muscle activation from step to step and across speeds. Next, consistent with the clinical notion of abnormal limb flexion-extension synergies post-stroke, we tested the hypothesis that subjects with post-stroke hemiparesis would have altered motor modules, leading to impaired walking performance. In post-stroke subjects (n = 55), a less complex coordination pattern was shown. Fewer modules were needed to account for muscle activation during walking at preferred speed compared with controls. Fewer modules resulted from merging of the modules observed in healthy controls, suggesting reduced independence of neural control signals. The number of modules was correlated to preferred walking speed, speed modulation, step length asymmetry, and propulsive asymmetry. Our results suggest a common modular organization of muscle coordination underlying walking in both healthy and post-stroke subjects. Identification of motor modules may lead to new insight into impaired locomotor coordination and the underlying neural systems.
Figures

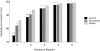


References
-
- Balasubramanian CK, Bowden MG, Neptune RR, Kautz SA. Relationship between step length asymmetry and walking performance in subjects with chronic hemiparesis. Arch Phys Med Rehabil 88: 43–49, 2007 - PubMed
-
- Bernstein NA. The Coordination and Regulation of Movements Oxford, UK: Pergamon Press, 1967
-
- Bowden MG, Balasubramanian CK, Neptune RR, Kautz SA. Anterior-posterior ground reaction forces as a measure of paretic leg contribution in hemiparetic walking. Stroke 37: 872–876, 2006 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

