Differential responses to Smith D autoantigen by mice with HLA-DR and HLA-DQ transgenes: dominant responses by HLA-DR3 transgenic mice with diversification of autoantibodies to small nuclear ribonucleoprotein, double-stranded DNA, and nuclear antigens
- PMID: 20007529
- PMCID: PMC2842881
- DOI: 10.4049/jimmunol.0902670
Differential responses to Smith D autoantigen by mice with HLA-DR and HLA-DQ transgenes: dominant responses by HLA-DR3 transgenic mice with diversification of autoantibodies to small nuclear ribonucleoprotein, double-stranded DNA, and nuclear antigens
Abstract
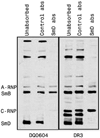
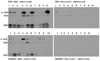
Anti-Smith (Sm) D autoantibodies are specific for systemic lupus erythematosus. In this investigation, the influence of HLA-D genes on immune responses to SmD was investigated. Mice with HLA-DR3, HLA-DR4, HLA-DQ0601, HLA-DQ0604, or HLA-DQ8 transgenes were immunized with recombinant SmD1, and their Ab responses were analyzed. Analysis by ELISA showed that all strains responded well to SmD. However, when synthetic SmD peptides were used as substrate, DR3 mice had the highest Ab response followed by DQ8, DQ0604, DQ0601, and DR4. A similar trend was observed in Western blot analysis using WEHI 7.1 cell lysate as the substrate, with the exception that DR4 mice did not generate detectable amounts of Abs. Only sera from DR3 and DQ0604 mice immunoprecipitated A-ribonucleoprotein (RNP), SmB, and SmD. Intermolecular epitope spreading to A-RNP and SmB was evident in DR3 and DQ0604 mice, as sera depleted of anti-SmD Abs were reactive with these proteins. DR3 mice also generated an immune response to C-RNP. Anti-nuclear Abs were detected in the majority of the DR3 mice, whereas moderate reactivities were seen in DQ0604 and DQ8 mice. Interestingly, only DR3 mice mounted an anti-dsDNA Ab response. Approximately half of the anti-dsDNA Abs were cross-reactive with SmD. Ab responses correlated with the strength of the T cell responses. Thus, HLA-DR3 appears to be the dominant HLA-D gene that determines the magnitude and quality of the anti-SmD immune response. In addition, our findings provide insights into the origin of the anti-dsDNA Abs often detected in patients with systemic lupus erythematosus.
Figures






Similar articles
-
Nature of T cell epitopes in lupus antigens and HLA-DR determines autoantibody initiation and diversification.Ann Rheum Dis. 2019 Mar;78(3):380-390. doi: 10.1136/annrheumdis-2018-214125. Epub 2018 Sep 25. Ann Rheum Dis. 2019. PMID: 30254034 Free PMC article.
-
HLA-DR3 restricted T cell epitope mimicry in induction of autoimmune response to lupus-associated antigen SmD.J Autoimmun. 2011 Nov;37(3):254-62. doi: 10.1016/j.jaut.2011.07.002. Epub 2011 Aug 24. J Autoimmun. 2011. PMID: 21868195 Free PMC article.
-
Immune responses to small nuclear ribonucleoproteins: antigen-dependent distinct B cell epitope spreading patterns in mice immunized with recombinant polypeptides of small nuclear ribonucleoproteins.J Immunol. 2002 May 15;168(10):5326-32. doi: 10.4049/jimmunol.168.10.5326. J Immunol. 2002. PMID: 11994491
-
Mechanisms of autoantibody diversification to SLE-related autoantigens.Ann N Y Acad Sci. 2003 Apr;987:91-8. doi: 10.1111/j.1749-6632.2003.tb06036.x. Ann N Y Acad Sci. 2003. PMID: 12727627 Review.
-
HLA and H2 class II transgenic mouse models to study susceptibility and protection in autoimmune thyroid disease.Autoimmunity. 2003 Sep-Nov;36(6-7):397-404. doi: 10.1080/08916930310001603028. Autoimmunity. 2003. PMID: 14669947 Review.
Cited by
-
Nature of T cell epitopes in lupus antigens and HLA-DR determines autoantibody initiation and diversification.Ann Rheum Dis. 2019 Mar;78(3):380-390. doi: 10.1136/annrheumdis-2018-214125. Epub 2018 Sep 25. Ann Rheum Dis. 2019. PMID: 30254034 Free PMC article.
-
Autoimmunity, end organ damage, and the origin of autoantibodies and autoreactive T cells in systemic lupus erythematosus.Discov Med. 2013 Feb;15(81):85-92. Discov Med. 2013. PMID: 23449110 Free PMC article. Review.
-
Genetic Polymorphisms of rs3077 and rs9277535 in HLA-DP associated with Systemic lupus erythematosus in a Chinese population.Sci Rep. 2017 Jan 17;7:39757. doi: 10.1038/srep39757. Sci Rep. 2017. PMID: 28094303 Free PMC article.
-
Immunogenetics of systemic lupus erythematosus: A comprehensive review.J Autoimmun. 2015 Nov;64:125-36. doi: 10.1016/j.jaut.2015.08.004. Epub 2015 Aug 29. J Autoimmun. 2015. PMID: 26324017 Free PMC article. Review.
-
HLA-DR3 restricted environmental epitopes from the bacterium Clostridium tetani have T cell cross-reactivity to the SLE-related autoantigen SmD.Front Immunol. 2022 Oct 31;13:928374. doi: 10.3389/fimmu.2022.928374. eCollection 2022. Front Immunol. 2022. PMID: 36389825 Free PMC article.
References
-
- Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277. - PubMed
-
- Ramos PS, Kelly JA, Gray-McGuire C, Bruner GR, Leiran AN, Meyer CM, Namjou B, Espe KJ, Ortmann WA, Reichlin M, Langefeld CD, James JA, Gaffney PM, Behrens TW, Harley JB, Moser KL. Familial aggregation and linkage analysis of autoantibody traits in pedigrees multiplex for systemic lupus erythematosus. Genes Immun. 2006;7:417–432. - PubMed
-
- Graham RR, Ortmann WA, Langefeld CD, Jawaheer D, Selby SA, Rodine PR, Baechler EC, Rohlf KE, Shark KB, Espe KJ, Green LE, Nair RP, Stuart PE, Elder JT, King RA, Moser KL, Gaffney PM, Bugawan TL, Erlich HA, Rich SS, Gregersen PK, Behrens TW. Visualizing human leukocyte antigen class II risk haplotypes in human systemic lupus erythematosus. Am. J. Hum. Genet. 2002;71:543–553. - PMC - PubMed
-
- Graham RR, Ortmann W, Rodine P, Espe K, Langefeld C, Lange E, Williams A, Beck S, Kyogoku C, Moser K, Gaffney P, Gregersen PK, Criswell LA, Harley JB, Behrens TW. Specific combinations of HLA-DR2 and DR3 class II haplotypes contribute graded risk for disease susceptibility and autoantibodies in human SLE. Eur. J. Hum. Genet. 2007;15:823–830. - PubMed
-
- Reveille JD. The genetic basis of autoantibody production. Autoimmun. Rev. 2006;5:389–398. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 AR045222/AR/NIAMS NIH HHS/United States
- K01 AR051391/AR/NIAMS NIH HHS/United States
- R01-AR30752/AR/NIAMS NIH HHS/United States
- R01 AI043248/AI/NIAID NIH HHS/United States
- K01-DK063065/DK/NIDDK NIH HHS/United States
- R01 AR049449/AR/NIAMS NIH HHS/United States
- K01 DK063065/DK/NIDDK NIH HHS/United States
- K01-AR051391/AR/NIAMS NIH HHS/United States
- R01-AI043248/AI/NIAID NIH HHS/United States
- P50-AR045222/AR/NIAMS NIH HHS/United States
- R01-AR049449/AR/NIAMS NIH HHS/United States
- R01 AR030752/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

