Cbfb/Runx1 repression-independent blockage of differentiation and accumulation of Csf2rb-expressing cells by Cbfb-MYH11
- PMID: 20007544
- PMCID: PMC2826765
- DOI: 10.1182/blood-2009-06-227413
Cbfb/Runx1 repression-independent blockage of differentiation and accumulation of Csf2rb-expressing cells by Cbfb-MYH11
Abstract
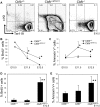
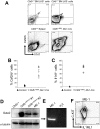
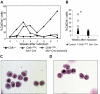
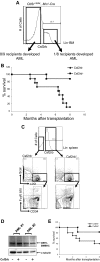
It is known that CBFB-MYH11, the fusion gene generated by inversion of chromosome 16 in human acute myeloid leukemia, is causative for oncogenic transformation. However, the mechanism by which CBFB-MYH11 initiates leukemogenesis is not clear. Previously published reports showed that CBFB-MYH11 dominantly inhibits RUNX1 and CBFB, and such inhibition has been suggested as the mechanism for leukemogenesis. Here we show that Cbfb-MYH11 caused Cbfb/Runx1 repression-independent defects in both primitive and definitive hematopoiesis. During primitive hematopoiesis, Cbfb-MYH11 delayed differentiation characterized by sustained expression of Gata2, Il1rl1, and Csf2rb, a phenotype not found in Cbfb and Runx1 knockout mice. Expression of Cbfb-MYH11 in the bone marrow induced the accumulation of abnormal progenitor-like cells expressing Csf2rb in preleukemic mice. The expression of all 3 genes was detected in most human and murine CBFB-MYH11(+) leukemia samples. Interestingly, Cbfb-MYH11(+) preleukemic progenitors and leukemia-initiating cells did not express Csf2rb, although the majority of leukemia cells in our Cbfb-MYH11 knockin mice were Csf2rb(+). Therefore Csf2rb can be used as a negative selection marker to enrich preleukemic progenitor cells and leukemia-initiating cells from Cbfb-MYH11 mice. These results suggest that Cbfb/Runx1 repression-independent activities contribute to leukemogenesis by Cbfb-MYH11.
Figures


References
-
- Le Beau MM, Larson RA, Bitter MA, Vardiman JW, Golomb HM, Rowley JD. Association of an inversion of chromosome 16 with abnormal marrow eosinophils in acute myelomonocytic leukemia: a unique cytogenetic-clinicopathological association. N Engl J Med. 1983;309(11):630–636. - PubMed
-
- Liu PP, Hajra A, Wijmenga C, Collins FS. Molecular pathogenesis of the chromosome 16 inversion in the M4Eo subtype of acute myeloid leukemia. Blood. 1995;85(9):2289–2302. - PubMed
-
- Liu P, Tarlé SA, Hajra A, et al. Fusion between transcription factor CBF beta/PEBP2 beta and a myosin heavy chain in acute myeloid leukemia. Science. 1993;261(5124):1041–1044. - PubMed
-
- Castilla LH, Garrett L, Adya N, et al. The fusion gene Cbfb-MYH11 blocks myeloid differentiation and predisposes mice to acute myelomonocytic leukaemia. Nat Genet. 1999;23(2):144–146. - PubMed
-
- Speck NA, Gilliland DG. Core-binding factors in haematopoiesis and leukaemia. Nat Rev Cancer. 2002;2(7):502–513. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

