Allogeneic hematopoietic stem-cell transplantation for sickle cell disease
- PMID: 20007560
- PMCID: PMC3627532
- DOI: 10.1056/NEJMoa0904971
Allogeneic hematopoietic stem-cell transplantation for sickle cell disease
Abstract
Background: Myeloablative allogeneic hematopoietic stem-cell transplantation is curative in children with sickle cell disease, but in adults the procedure is unduly toxic. Graft rejection and graft-versus-host disease (GVHD) are additional barriers to its success. We performed nonmyeloablative stem-cell transplantation in adults with sickle cell disease.
Methods: Ten adults (age range, 16 to 45 years) with severe sickle cell disease underwent nonmyeloablative transplantation with CD34+ peripheral-blood stem cells, mobilized by granulocyte colony-stimulating factor (G-CSF), which were obtained from HLA-matched siblings. The patients received 300 cGy of total-body irradiation plus alemtuzumab before transplantation, and sirolimus was administered afterward.
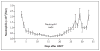
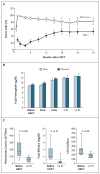
Results: All 10 patients were alive at a median follow-up of 30 months after transplantation (range, 15 to 54). Nine patients had long-term, stable donor lymphohematopoietic engraftment at levels that sufficed to reverse the sickle cell disease phenotype. Mean (+/-SE) donor-recipient chimerism for T cells (CD3+) and myeloid cells (CD14+15+) was 53.3+/-8.6% and 83.3+/-10.3%, respectively, in the nine patients whose grafts were successful. Hemoglobin values before transplantation and at the last follow-up assessment were 9.0+/-0.3 and 12.6+/-0.5 g per deciliter, respectively. Serious adverse events included the narcotic-withdrawal syndrome and sirolimus-associated pneumonitis and arthralgia. Neither acute nor chronic GVHD developed in any patient.
Conclusions: A protocol for nonmyeloablative allogeneic hematopoietic stem-cell transplantation that includes total-body irradiation and treatment with alemtuzumab and sirolimus can achieve stable, mixed donor-recipient chimerism and reverse the sickle cell phenotype. (ClinicalTrials.gov number, NCT00061568.)
2009 Massachusetts Medical Society
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
Comment in
-
Hematopoietic stem-cell transplantation for adults with sickle cell disease.N Engl J Med. 2009 Dec 10;361(24):2380-1. doi: 10.1056/NEJMe0908574. N Engl J Med. 2009. PMID: 20007564 No abstract available.
-
Stem-cell transplantation for sickle cell disease.N Engl J Med. 2010 Mar 11;362(10):955; author reply 956. doi: 10.1056/NEJMc1000134. N Engl J Med. 2010. PMID: 20220194 No abstract available.
-
Stem-cell transplantation for sickle cell disease.N Engl J Med. 2010 Mar 11;362(10):955-6; author reply 956. N Engl J Med. 2010. PMID: 20225347 No abstract available.
References
-
- Pauling L, Itano HA, Singer SJ, Wells IC. Sickle cell anemia, a molecular disease. Science. 1949;110:543–8. - PubMed
-
- Ingram VM. Gene mutations in human haemoglobin: the chemical difference between normal and sickle cell haemoglobin. Nature. 1957;180:326–8. - PubMed
-
- Walters MC, Patience M, Leisenring W, et al. Bone marrow transplantation for sickle cell disease. N Engl J Med. 1996;335:369–76. - PubMed
-
- Vermylen C, Cornu G, Ferster A, et al. Haematopoietic stem cell transplantation for sickle cell anaemia: the first 50 patients transplanted in Belgium. Bone Marrow Transplant. 1998;22:1–6. - PubMed
-
- Bernaudin F, Socie G, Kuentz M, et al. Long-term results of related myeloablative stem-cell transplantation to cure sickle cell disease. Blood. 2007;110:2749–56. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
