Inflation and long-term maintenance of CD8 T cells responding to a latent herpesvirus depend upon establishment of latency and presence of viral antigens
- PMID: 20007576
- PMCID: PMC4161222
- DOI: 10.4049/jimmunol.0801117
Inflation and long-term maintenance of CD8 T cells responding to a latent herpesvirus depend upon establishment of latency and presence of viral antigens
Abstract
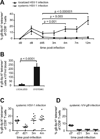
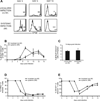
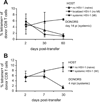
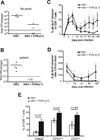
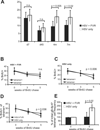
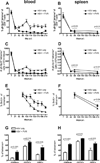
Following the priming and contraction phases of the T cell response, latent persistent herpesviruses lead to an accumulation of large pools of virus-specific CD8 T cells, also known as memory inflation (MI). The mechanism of this inflation is incompletely understood, largely because the molecular reactivation of these viruses in vivo and its impact upon T cell biology have not been resolved in mice, and because the relevant observations in humans remain, by necessity, correlative. Understanding these processes is essential from the standpoint of the proposed critical role for latent herpesviruses in aging of the immune system. We studied the causes of memory CD8 T cell accumulation following systemic HSV-1 administration as a model of widespread latent viral infection in humans. A direct role of viral latency and Ag-specific restimulation in driving the accumulation and maintenance of inflated CD8 T cells and a strongly suggested role of viral reactivation in that process were shown by the following: 1) lack of MI in the absence of established latency; 2) prevention or delay of MI with drugs that curtail viral replication; and 3) abrogation of MI by the transfer of inflated T cells into a virus-free environment. These results strongly suggest that periodic, subclinical reactivations of a latent persistent virus cause dysregulation of memory CD8 T cell homeostasis, similar to the one in humans. Moreover, results with antiviral drugs suggest that this approach could be considered as a treatment modality for maintaining T cell diversity and/or function in old age.
Figures


References
-
- Pawelec G, Akbar A, Caruso C, Effros R, Grubeck-Loebenstein B, Wikby A. Is immunosenescence infectious? Trends Immunol. 2004;25:406–410. - PubMed
-
- Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, Sleath PR, Grabstein KH, Hosken NA, Kern F, Nelson JA, Picker LJ. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med. 2005;202:673–685. - PMC - PubMed
-
- Munks MW, Gold MC, Zajac AL, Doom CM, Morello CS, Spector DH, Hill AB. Genome-wide analysis reveals a highly diverse CD8 T cell response to murine cytomegalovirus. J Immunol. 2006;176:3760–3766. - PubMed
-
- Wikby A, Ferguson F, Forsey R, Thompson J, Strindhall J, Lofgren S, Nilsson BO, Ernerudh J, Pawelec G, Johansson B. An immune risk phenotype, cognitive impairment, and survival in very late life: impact of allostatic load in Swedish octogenarian and nonagenarian humans. J Gerontol A Biol Sci Med Sci. 2005;60:556–565. - PubMed
-
- Mocarski ES, C CT. Cytomegaloviruses and their replication. In: Knipe DM, Howley PM, editors. Fields - Virology. Philadelphia: Lippincot Williams & Wilkins; 2001. pp. 2629–2674.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

