Differential role of the Fas/Fas ligand apoptotic pathway in inflammation and lung fibrosis associated with reovirus 1/L-induced bronchiolitis obliterans organizing pneumonia and acute respiratory distress syndrome
- PMID: 20007588
- PMCID: PMC2814248
- DOI: 10.4049/jimmunol.0901958
Differential role of the Fas/Fas ligand apoptotic pathway in inflammation and lung fibrosis associated with reovirus 1/L-induced bronchiolitis obliterans organizing pneumonia and acute respiratory distress syndrome
Abstract
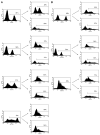
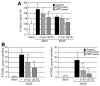
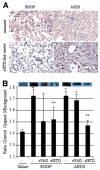
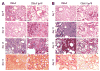
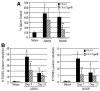
Bronchiolitis obliterans organizing pneumonia (BOOP) and acute respiratory distress syndrome (ARDS) are two clinically and histologically distinct syndromes sharing the presence of an inflammatory and fibrotic component. Apoptosis via the Fas/Fas ligand (FasL) pathway plays an important role in the development of acute lung injury and fibrosis characteristic of these and other pulmonary inflammatory and fibrotic syndromes. We evaluated the role of apoptosis via the Fas/FasL pathway in the development of pulmonary inflammation and fibrosis in reovirus 1/L-induced BOOP and ARDS. CBA/J mice were intranasally inoculated with saline, 1 x 10(6) (BOOP), or 1 x 10(7) (ARDS) PFU reovirus 1/L, and evaluated at various days postinoculation for in situ apoptosis by TUNEL analysis and Fas/FasL expression. Our results demonstrate the presence of apoptotic cells and up-regulation of Fas/FasL expression in alveolar epithelium and in infiltrating cells during the inflammatory and fibrotic stages of both reovirus 1/L-induced ARDS and BOOP. Treatment of mice with the caspase 8 inhibitor, zIETD-fmk, inhibited apoptosis, inflammation, and fibrotic lesion development in reovirus 1/L-induced BOOP and ARDS. However, CBA/KlJms-Fas(lpr-cg)/J mice, which carry a point mutation in the Fas cytoplasmic region that abolishes the ability of Fas to transduce an apoptotic signal, do not develop pulmonary inflammation and fibrotic lesions associated with reovirus 1/L-induced BOOP, but still develop inflammation and fibrotic lesions associated with reovirus 1/L-induced ARDS. These results suggest a differential role for the Fas/FasL apoptotic pathway in the development of inflammation and fibrotic lesions associated with BOOP and ARDS.
Conflict of interest statement
Figures

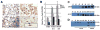

Similar articles
-
Differential role for T cells in the development of fibrotic lesions associated with reovirus 1/L-induced bronchiolitis obliterans organizing pneumonia versus Acute Respiratory Distress Syndrome.Am J Respir Cell Mol Biol. 2003 Feb;28(2):208-17. doi: 10.1165/rcmb.4891. Am J Respir Cell Mol Biol. 2003. PMID: 12540488
-
Respiratory reovirus 1/L induction of intraluminal fibrosis, a model of bronchiolitis obliterans organizing pneumonia, is dependent on T lymphocytes.Am J Pathol. 2003 Oct;163(4):1467-79. doi: 10.1016/S0002-9440(10)63504-3. Am J Pathol. 2003. PMID: 14507654 Free PMC article.
-
Respiratory reovirus 1/L induction of intraluminal fibrosis. A model for the study of bronchiolitis obliterans organizing pneumonia.Am J Pathol. 1997 Jun;150(6):2243-54. Am J Pathol. 1997. PMID: 9176413 Free PMC article.
-
Bronchiolitis obliterans. Organizing pneumonia.Verh Dtsch Ges Pathol. 2002;86:101-6. Verh Dtsch Ges Pathol. 2002. PMID: 12647357 Review.
-
The central role of Fas-ligand cell signaling in inflammatory lung diseases.J Cell Mol Med. 2004 Jul-Sep;8(3):285-93. doi: 10.1111/j.1582-4934.2004.tb00318.x. J Cell Mol Med. 2004. PMID: 15491504 Free PMC article. Review.
Cited by
-
Organizing pneumonia after stereotactic ablative radiotherapy of the lung.Radiat Oncol. 2012 Aug 1;7:123. doi: 10.1186/1748-717X-7-123. Radiat Oncol. 2012. PMID: 22853821 Free PMC article.
-
The Basic Science and Molecular Mechanisms of Lung Injury and Acute Respiratory Distress Syndrome.Int Anesthesiol Clin. 2018 Winter;56(1):1-25. doi: 10.1097/AIA.0000000000000177. Int Anesthesiol Clin. 2018. PMID: 29227309 Free PMC article. Review. No abstract available.
-
Catalpol Protects Against Pulmonary Fibrosis Through Inhibiting TGF-β1/Smad3 and Wnt/β-Catenin Signaling Pathways.Front Pharmacol. 2021 Jan 29;11:594139. doi: 10.3389/fphar.2020.594139. eCollection 2020. Front Pharmacol. 2021. PMID: 33584272 Free PMC article.
-
IL-13 signaling via IL-13Rα2 triggers TGF-β1-dependent allograft fibrosis.Transplant Res. 2013 Oct 22;2(1):16. doi: 10.1186/2047-1440-2-16. Transplant Res. 2013. PMID: 24143891 Free PMC article.
-
Lugrandoside attenuates LPS-induced acute respiratory distress syndrome by anti-inflammation and anti-apoptosis in mice.Am J Transl Res. 2016 Dec 15;8(12):5557-5568. eCollection 2016. Am J Transl Res. 2016. PMID: 28078026 Free PMC article.
References
-
- Cooper JA., Jr Pulmonary fibrosis: pathways are slowly coming into light. Am J Resp Cell Mol Biol. 2000;22:520–523. - PubMed
-
- Kuwano K, Hagimoto N, Hara N. Molecular mechanisms of pulmonary fibrosis and current treatment. Curr Mol Med. 2001;1:551–573. - PubMed
-
- Katzenstein AL, Mukhopadhyay S, Myers JL. Diagnosis of usual interstitial pneumonia and distinction from other fibrosing interstitial lung diseases. Hum Pathol. 2008;39:1275–1294. - PubMed
-
- Afshar K, Sharma OP. Interstitial lung disease: trials and tribulations. Curr Opin Pulm Med. 2008;14:427–433. - PubMed
-
- Drakopanagiotakis F, Polychronopoulos V, Judson MA. Organizing pneumonia. Am J Med Sci. 2008;335:34–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

