RNA helicase A modulates translation of HIV-1 and infectivity of progeny virions
- PMID: 20007598
- PMCID: PMC2836548
- DOI: 10.1093/nar/gkp1075
RNA helicase A modulates translation of HIV-1 and infectivity of progeny virions
Abstract
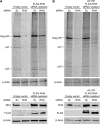
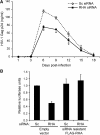
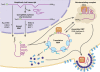
Retroviruses rely on host RNA-binding proteins to modulate various steps in their replication. Previously several animal retroviruses were determined to mediate Dhx9/RNA helicase A (RHA) interaction with a 5' terminal post-transcriptional control element (PCE) for efficient translation. Herein PCE reporter assays determined HTLV-1 and HIV-1 RU5 confer orientation-dependent PCE activity. The effect of Dhx9/RHA down-regulation and rescue with siRNA-resistant RHA on expression of HIV-1(NL4-3) provirus determined that RHA is necessary for efficient HIV-1 RNA translation and requires ATPase-dependent helicase function. Quantitative analysis determined HIV-1 RNA steady-state and cytoplasmic accumulation were not reduced; rather the translational activity of viral RNA was reduced. Western blotting determined that RHA-deficient virions assemble with Lys-tRNA synthetase, exhibit processed reverse transcriptase and contain similar level of viral RNA, but they are poorly infectious on primary lymphocytes and HeLa cells. The results demonstrate RHA is an important host factor within the virus-producer cell and within the viral particle. The identification of RHA-dependent PCE activity in cellular junD RNA and in six of seven genera of Retroviridae suggests conservation of this translational control mechanism among vertebrates, and convergent evolution of Retroviridae to utilize this host mechanism.
Figures


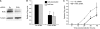



Similar articles
-
Virion-associated, host-derived DHX9/RNA helicase A enhances the processivity of HIV-1 reverse transcriptase on genomic RNA.J Biol Chem. 2019 Jul 26;294(30):11473-11485. doi: 10.1074/jbc.RA119.007679. Epub 2019 Jun 7. J Biol Chem. 2019. PMID: 31175158 Free PMC article.
-
DHX9/RHA Binding to the PBS-Segment of the Genomic RNA during HIV-1 Assembly Bolsters Virion Infectivity.J Mol Biol. 2016 Jun 5;428(11):2418-2429. doi: 10.1016/j.jmb.2016.04.011. Epub 2016 Apr 21. J Mol Biol. 2016. PMID: 27107641 Free PMC article.
-
RNA helicase A interacts with divergent lymphotropic retroviruses and promotes translation of human T-cell leukemia virus type 1.Nucleic Acids Res. 2007;35(8):2629-42. doi: 10.1093/nar/gkm124. Epub 2007 Apr 10. Nucleic Acids Res. 2007. PMID: 17426138 Free PMC article.
-
tRNA(Lys3): the primer tRNA for reverse transcription in HIV-1.IUBMB Life. 2002 Feb;53(2):107-14. doi: 10.1080/15216540211469. IUBMB Life. 2002. PMID: 12049192 Review.
-
The selective packaging and annealing of primer tRNALys3 in HIV-1.Curr HIV Res. 2004 Apr;2(2):163-75. doi: 10.2174/1570162043484988. Curr HIV Res. 2004. PMID: 15078180 Review.
Cited by
-
Sensitivity of yeast to lithium chloride connects the activity of YTA6 and YPR096C to translation of structured mRNAs.PLoS One. 2020 Jul 8;15(7):e0235033. doi: 10.1371/journal.pone.0235033. eCollection 2020. PLoS One. 2020. PMID: 32639961 Free PMC article.
-
General and Target-Specific DExD/H RNA Helicases in Eukaryotic Translation Initiation.Int J Mol Sci. 2020 Jun 20;21(12):4402. doi: 10.3390/ijms21124402. Int J Mol Sci. 2020. PMID: 32575790 Free PMC article. Review.
-
DExD/H-box RNA helicases as mediators of anti-viral innate immunity and essential host factors for viral replication.Biochim Biophys Acta. 2013 Aug;1829(8):854-65. doi: 10.1016/j.bbagrm.2013.03.012. Epub 2013 Apr 6. Biochim Biophys Acta. 2013. PMID: 23567047 Free PMC article. Review.
-
Anomalous HIV-1 RNA, How Cap-Methylation Segregates Viral Transcripts by Form and Function.Viruses. 2022 Apr 29;14(5):935. doi: 10.3390/v14050935. Viruses. 2022. PMID: 35632676 Free PMC article. Review.
-
DHX9 pairs with IPS-1 to sense double-stranded RNA in myeloid dendritic cells.J Immunol. 2011 Nov 1;187(9):4501-8. doi: 10.4049/jimmunol.1101307. Epub 2011 Sep 28. J Immunol. 2011. PMID: 21957149 Free PMC article.
References
-
- Hartman TR, Qian S, Bolinger C, Fernandez S, Schoenberg DR, Boris-Lawrie K. RNA helicase A is necessary for translation of selected messenger RNAs. Nat. Struct. Mol. Biol. 2006;13:509–516. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

