Rad26p, a transcription-coupled repair factor, is recruited to the site of DNA lesion in an elongating RNA polymerase II-dependent manner in vivo
- PMID: 20007604
- PMCID: PMC2836574
- DOI: 10.1093/nar/gkp1147
Rad26p, a transcription-coupled repair factor, is recruited to the site of DNA lesion in an elongating RNA polymerase II-dependent manner in vivo
Abstract
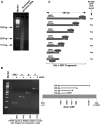
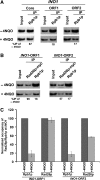
Rad26p, a yeast homologue of human Cockayne syndrome B with an ATPase activity, plays a pivotal role in stimulating DNA repair at the coding sequences of active genes. On the other hand, DNA repair at inactive genes or silent areas of the genome is not regulated by Rad26p. However, how Rad26p recognizes DNA lesions at the actively transcribing genes to facilitate DNA repair is not clearly understood in vivo. Here, we show that Rad26p associates with the coding sequences of genes in a transcription-dependent manner, but independently of DNA lesions induced by 4-nitroquinoline-1-oxide in Saccharomyces cerevisiae. Further, histone H3 lysine 36 methylation that occurs at the active coding sequence stimulates the recruitment of Rad26p. Intriguingly, we find that Rad26p is recruited to the site of DNA lesion in an elongating RNA polymerase II-dependent manner. However, Rad26p does not recognize DNA lesions in the absence of active transcription. Together, these results provide an important insight as to how Rad26p is delivered to the damage sites at the active, but not inactive, genes to stimulate repair in vivo, shedding much light on the early steps of transcription-coupled repair in living eukaryotic cells.
Figures





Similar articles
-
Rad26p, a transcription-coupled repair factor, promotes the eviction and prevents the reassociation of histone H2A-H2B dimer during transcriptional elongation in vivo.Biochemistry. 2012 Jul 31;51(30):5873-5. doi: 10.1021/bi3005768. Epub 2012 Jul 20. Biochemistry. 2012. PMID: 22794311 Free PMC article.
-
Rad26p regulates the occupancy of histone H2A-H2B dimer at the active genes in vivo.Nucleic Acids Res. 2012 Apr;40(8):3348-63. doi: 10.1093/nar/gkr1244. Epub 2011 Dec 22. Nucleic Acids Res. 2012. PMID: 22199252 Free PMC article.
-
Methylation of histone H3 K4 mediates association of the Isw1p ATPase with chromatin.Mol Cell. 2003 Nov;12(5):1325-32. doi: 10.1016/s1097-2765(03)00438-6. Mol Cell. 2003. PMID: 14636589
-
A site to remember: H3K36 methylation a mark for histone deacetylation.Mutat Res. 2007 May 1;618(1-2):130-4. doi: 10.1016/j.mrfmmm.2006.08.014. Epub 2007 Jan 21. Mutat Res. 2007. PMID: 17346757 Review.
-
Facilitators and Repressors of Transcription-coupled DNA Repair in Saccharomyces cerevisiae.Photochem Photobiol. 2017 Jan;93(1):259-267. doi: 10.1111/php.12655. Epub 2016 Nov 30. Photochem Photobiol. 2017. PMID: 27796045 Review.
Cited by
-
Histone H3 lysine 36 methyltransferase mobilizes NER factors to regulate tolerance against alkylation damage in fission yeast.Nucleic Acids Res. 2018 Jun 1;46(10):5061-5074. doi: 10.1093/nar/gky245. Nucleic Acids Res. 2018. PMID: 29635344 Free PMC article.
-
TOR Facilitates the Targeting of the 19S Proteasome Subcomplex To Enhance Transcription Complex Assembly at the Promoters of the Ribosomal Protein Genes.Mol Cell Biol. 2018 Jun 28;38(14):e00469-17. doi: 10.1128/MCB.00469-17. Print 2018 Jul 15. Mol Cell Biol. 2018. PMID: 29712756 Free PMC article.
-
Molecular basis of transcriptional fidelity and DNA lesion-induced transcriptional mutagenesis.DNA Repair (Amst). 2014 Jul;19:71-83. doi: 10.1016/j.dnarep.2014.03.024. Epub 2014 Apr 21. DNA Repair (Amst). 2014. PMID: 24767259 Free PMC article. Review.
-
Regulation of the Rhp26ERCC6/CSB chromatin remodeler by a novel conserved leucine latch motif.Proc Natl Acad Sci U S A. 2014 Dec 30;111(52):18566-71. doi: 10.1073/pnas.1420227112. Epub 2014 Dec 15. Proc Natl Acad Sci U S A. 2014. PMID: 25512493 Free PMC article.
-
Rad26p, a transcription-coupled repair factor, promotes the eviction and prevents the reassociation of histone H2A-H2B dimer during transcriptional elongation in vivo.Biochemistry. 2012 Jul 31;51(30):5873-5. doi: 10.1021/bi3005768. Epub 2012 Jul 20. Biochemistry. 2012. PMID: 22794311 Free PMC article.
References
-
- Sancar A, Lindsey-Boltz LA, Unsal-Kaccmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu. Rev. Biochem. 2004;73:39–85. - PubMed
-
- Bootsma D, Kraemer KH, Cleaver JE, Hoeijmakers JHJ. Nucleotide excision repair syndromes: xeroderma pigmentosum, Cockayne syndrome, and trichothiodystrophy. In: Vogelstein B, Kinzler KW, editors. The Genetic Basis of Human Cancer. 2nd edn. New York: McGraw-Hill; 2002. pp. 211–237.
-
- Cooper PK, Nouspikel T, Clarkson SG, Leadon SA. Defective transcription-coupled repair of oxidative base damage in Cockayne syndrome patients from XP group G. Science. 1997;275:990–993. - PubMed
-
- Hasty P, Campisi J, Hoeijmakers J, van Steeg H, Vijg J. Aging and genome maintenance: lessons from the mouse. Science. 2003;299:1355–1359. - PubMed
-
- Sands AT, Abuin A, Sanchez A, Conti CJ, Bradley A. High susceptibility to ultraviolet-induced carcinogenesis in mice lacking XPC. Nature. 1995;377:162–165. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

