BCR-ABL gene expression is required for its mutations in a novel KCL-22 cell culture model for acquired resistance of chronic myelogenous leukemia
- PMID: 20007699
- PMCID: PMC2836111
- DOI: 10.1074/jbc.M109.039206
BCR-ABL gene expression is required for its mutations in a novel KCL-22 cell culture model for acquired resistance of chronic myelogenous leukemia
Abstract
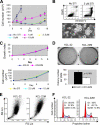
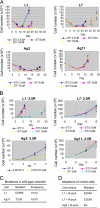
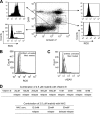
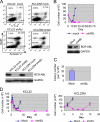
Acquired resistance through genetic mutations is a common phenomenon in several cancer therapies using molecularly targeted drugs, best exemplified by the BCR-ABL inhibitor imatinib in treating chronic myelogenous leukemia (CML). Overcoming acquired resistance is a daunting therapeutic challenge, and little is known about how these mutations evolve. To facilitate understanding the resistance mechanisms, we developed a novel culture model for CML acquired resistance in which the CML cell line KCL-22, following initial response to imatinib, develops resistant T315I BCR-ABL mutation. We demonstrate that the emergence of BCR-ABL mutations do not require pre-existing BCR-ABL mutations derived from the original patient as the subclones of KCL-22 cells can form various BCR-ABL mutations upon imatinib treatment. BCR-ABL mutation rates vary from cell clone to clone and passages, in contrast to the relatively stable mutation rate of the hypoxanthine-guanine phosphoribosyltransferase gene. Strikingly, development of BCR-ABL mutations depends on its gene expression because BCR-ABL knockdown completely blocks KCL-22 cell relapse on imatinib and acquisition of mutations. We further show that the endogenous BCR-ABL locus has significantly higher mutagenesis potential than the transduced randomly integrated BCR-ABL cDNA. Our study suggests important roles of BCR-ABL gene expression and its native chromosomal locus for acquisition of BCR-ABL mutations and provides a new tool for further studying resistance mechanisms.
Figures




References
-
- Deininger M. W., Druker B. J. (2003) Pharmacol. Rev. 55, 401–423 - PubMed
-
- Gorre M. E., Mohammed M., Ellwood K., Hsu N., Paquette R., Rao P. N., Sawyers C. L. (2001) Science 293, 876–880 - PubMed
-
- Shah N. P., Nicoll J. M., Nagar B., Gorre M. E., Paquette R. L., Kuriyan J., Sawyers C. L. (2002) Cancer Cell 2, 117–125 - PubMed
-
- Branford S., Rudzki Z., Walsh S., Grigg A., Arthur C., Taylor K., Herrmann R., Lynch K. P., Hughes T. P. (2002) Blood 99, 3472–3475 - PubMed
-
- Weisberg E., Manley P. W., Breitenstein W., Brüggen J., Cowan-Jacob S. W., Ray A., Huntly B., Fabbro D., Fendrich G., Hall-Meyers E., Kung A. L., Mestan J., Daley G. Q., Callahan L., Catley L., Cavazza C., Azam M., Mohammed A., Neuberg D., Wright R. D., Gilliland D. G., Griffin J. D. (2005) Cancer Cell 7, 129–141 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

