Niemann-Pick C1 functions independently of Niemann-Pick C2 in the initial stage of retrograde transport of membrane-impermeable lysosomal cargo
- PMID: 20007703
- PMCID: PMC2836102
- DOI: 10.1074/jbc.M109.037622
Niemann-Pick C1 functions independently of Niemann-Pick C2 in the initial stage of retrograde transport of membrane-impermeable lysosomal cargo
Abstract
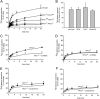
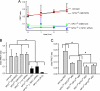
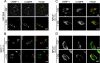
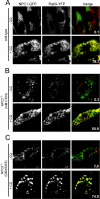
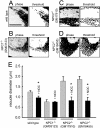
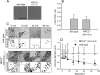
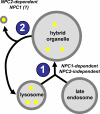
The rare neurodegenerative disease Niemann-Pick Type C (NPC) results from mutations in either NPC1 or NPC2, which are membrane-bound and soluble lysosomal proteins, respectively. Previous studies have shown that mutations in either protein result in biochemically indistinguishable phenotypes, most notably the hyper-accumulation of cholesterol and other cargo in lysosomes. We comparatively evaluated the kinetics of [(3)H]dextran release from lysosomes of wild type, NPC1, NPC2, and NPC1/NPC2 pseudo-double mutant cells and found significant differences between all cell types examined. Specifically, NPC1 or NPC2 mutant fibroblasts treated with NPC1 or NPC2 siRNA (to create NPC1/NPC2 pseudo-double mutants) secreted dextran less efficiently than did either NPC1 or NPC2 single mutant cell lines, suggesting that the two proteins may work independently of one another in the egress of membrane-impermeable lysosomal cargo. To investigate the basis for these differences, we examined the role of NPC1 and NPC2 in the retrograde fusion of lysosomes with late endosomes to create so-called hybrid organelles, which is believed to be the initial step in the egress of cargo from lysosomes. We show here that cells with mutated NPC1 have significantly reduced rates of late endosome/lysosome fusion relative to wild type cells, whereas cells with mutations in NPC2 have rates that are similar to those observed in wild type cells. Instead of being involved in hybrid organelle formation, we show that NPC2 is required for efficient membrane fission events from nascent hybrid organelles, which is thought to be required for the reformation of lysosomes and the release of lysosomal cargo-containing membrane vesicles. Collectively, these results suggest that NPC1 and NPC2 can function independently of one another in the egress of certain membrane-impermeable lysosomal cargo.
Figures
Similar articles
-
ABCA1-dependent mobilization of lysosomal cholesterol requires functional Niemann-Pick C2 but not Niemann-Pick C1 protein.Biochim Biophys Acta. 2012 Mar;1821(3):396-404. doi: 10.1016/j.bbalip.2011.11.013. Epub 2011 Dec 10. Biochim Biophys Acta. 2012. PMID: 22179027
-
Differential trafficking of the Niemann-Pick C1 and 2 proteins highlights distinct roles in late endocytic lipid trafficking.Acta Paediatr Suppl. 2003 Dec;92(443):63-73; discussion 45. doi: 10.1111/j.1651-2227.2003.tb00224.x. Acta Paediatr Suppl. 2003. PMID: 14989468
-
NPC2 facilitates bidirectional transfer of cholesterol between NPC1 and lipid bilayers, a step in cholesterol egress from lysosomes.Proc Natl Acad Sci U S A. 2008 Oct 7;105(40):15287-92. doi: 10.1073/pnas.0807328105. Epub 2008 Sep 4. Proc Natl Acad Sci U S A. 2008. PMID: 18772377 Free PMC article.
-
Niemann-Pick C disease and mobilization of lysosomal cholesterol by cyclodextrin.J Lipid Res. 2014 Aug;55(8):1609-21. doi: 10.1194/jlr.R047837. Epub 2014 Mar 24. J Lipid Res. 2014. PMID: 24664998 Free PMC article. Review.
-
Function of the Niemann-Pick type C proteins and their bypass by cyclodextrin.Curr Opin Lipidol. 2011 Jun;22(3):204-9. doi: 10.1097/MOL.0b013e3283453e69. Curr Opin Lipidol. 2011. PMID: 21412152 Review.
Cited by
-
Somatic cell plasticity and Niemann-Pick type C2 protein: fibroblast activation.J Biol Chem. 2011 Jan 21;286(3):2078-87. doi: 10.1074/jbc.M110.135897. Epub 2010 Nov 17. J Biol Chem. 2011. PMID: 21084287 Free PMC article.
-
APP overexpression in the absence of NPC1 exacerbates metabolism of amyloidogenic proteins of Alzheimer's disease.Hum Mol Genet. 2015 Dec 15;24(24):7132-50. doi: 10.1093/hmg/ddv413. Epub 2015 Oct 3. Hum Mol Genet. 2015. PMID: 26433932 Free PMC article.
-
The Effects of Extracellular Serum Concentration on APP Processing in Npc1-Deficient APP-Overexpressing N2a Cells.Mol Neurobiol. 2018 Jul;55(7):5757-5766. doi: 10.1007/s12035-017-0799-5. Epub 2017 Oct 19. Mol Neurobiol. 2018. PMID: 29052144
-
Recent neuroimaging, neurophysiological, and neuropathological advances for the understanding of NPC.F1000Res. 2018 Feb 15;7:194. doi: 10.12688/f1000research.12361.1. eCollection 2018. F1000Res. 2018. PMID: 29511534 Free PMC article. Review.
-
The cell biology of disease: lysosomal storage disorders: the cellular impact of lysosomal dysfunction.J Cell Biol. 2012 Nov 26;199(5):723-34. doi: 10.1083/jcb.201208152. J Cell Biol. 2012. PMID: 23185029 Free PMC article. Review.
References
-
- Liscum L., Sturley S. L. (2004) Biochim. Biophys. Acta 1685, 22–27 - PubMed
-
- Carstea E. D., Morris J. A., Coleman K. G., Loftus S. K., Zhang D., Cummings C., Gu J., Rosenfeld M. A., Pavan W. J., Krizman D. B., Nagle J., Polymeropoulos M. H., Sturley S. L., Ioannou Y. A., Higgins M. E., Comly M., Cooney A., Brown A., Kaneski C. R., Blanchette-Mackie E. J., Dwyer N. K., Neufeld E. B., Chang T. Y., Liscum L., Strauss J. F., 3rd, Ohno K., Zeigler M., Carmi R., Sokol J., Markie D., O'Neill R. R., van Diggelen O. P., Elleder M., Patterson M. C., Brady R. O., Vanier M. T., Pentchev P. G., Tagle D. A. (1997) Science 277, 228–231 - PubMed
-
- Naureckiene S., Sleat D. E., Lackland H., Fensom A., Vanier M. T., Wattiaux R., Jadot M., Lobel P. (2000) Science 290, 2298–2301 - PubMed
-
- Pentchev P. G., Comly M. E., Kruth H. S., Tokoro T., Butler J., Sokol J., Filling-Katz M., Quirk J. M., Marshall D. C., Patel S. (1987) FASEB J. 1, 40–45 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

