The intracellular loop of Orai1 plays a central role in fast inactivation of Ca2+ release-activated Ca2+ channels
- PMID: 20007711
- PMCID: PMC2836109
- DOI: 10.1074/jbc.M109.072736
The intracellular loop of Orai1 plays a central role in fast inactivation of Ca2+ release-activated Ca2+ channels
Abstract
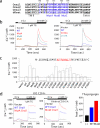
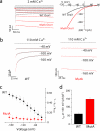
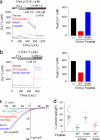
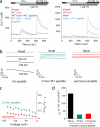
Store-operated Ca(2+) entry (SOCE) due to activation of Ca(2+) release-activated Ca(2+) (CRAC) channels leads to sustained elevation of cytoplasmic Ca(2+) and activation of lymphocytes. CRAC channels consisting of four pore-forming Orai1 subunits are activated by STIM1, an endoplasmic reticulum Ca(2+) sensor that senses intracellular store depletion and migrates to plasma membrane proximal regions to mediate SOCE. One of the fundamental properties of CRAC channels is their Ca(2+)-dependent fast inactivation. To identify the domains of Orai1 involved in fast inactivation, we have mutated residues in the Orai1 intracellular loop linking transmembrane segment II to III. Mutation of four residues, V(151)SNV(154), at the center of the loop (MutA) abrogated fast inactivation, leading to increased SOCE as well as higher CRAC currents. Point mutation analysis identified five key amino acids, N(153)VHNL(157), that increased SOCE in Orai1 null murine embryonic fibroblasts. Expression or direct application of a peptide comprising the entire intracellular loop or the sequence N(153)VHNL(157) blocked CRAC currents from both wild type (WT) and MutA Orai1. A peptide incorporating the MutA mutations had no blocking effect. Concatenated Orai1 constructs with four MutA monomers exhibited high CRAC currents lacking fast inactivation. Reintroduction of a single WT monomer (MutA-MutA-MutA-WT) was sufficient to fully restore fast inactivation, suggesting that only a single intracellular loop can block the channel. These data suggest that the intracellular loop of Orai1 acts as an inactivation particle, which is stabilized in the ion permeation pathway by the N(153)VHNL(157) residues. These results along with recent reports support a model in which the N terminus and the selectivity filter of Orai1 as well as STIM1 act in concert to regulate the movement of the intracellular loop and evoke fast inactivation.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

