Inflammation and adipose tissue macrophages in lipodystrophic mice
- PMID: 20007767
- PMCID: PMC2806777
- DOI: 10.1073/pnas.0905310107
Inflammation and adipose tissue macrophages in lipodystrophic mice
Abstract
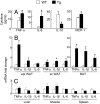
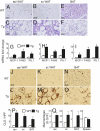
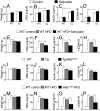
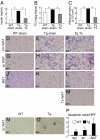
Lipodystrophy and obesity are opposites in terms of a deficiency versus excess of adipose tissue mass, yet these conditions are accompanied by similar metabolic consequences, including insulin resistance, dyslipidemia, hepatic steatosis, and increased risk for diabetes and atherosclerosis. Hepatic and myocellular steatosis likely contribute to metabolic dysregulation in both states. Inflammation and macrophage infiltration into adipose tissue also appear to participate in the pathogenesis of obesity-induced insulin resistance, but their contributions to lipodystrophy-induced insulin resistance have not been evaluated. We used aP2-nSREBP-1c transgenic (Tg) mice, an established model of lipodystrophy, to ask this question. Circulating cytokine elevations suggested systemic inflammation but even more dramatic was the number of infiltrating macrophages in all white and brown adipose tissue depots of the Tg mice; in contrast, there was no evidence of inflammatory infiltrates or responses in any other tissue including liver. Despite there being overt evidence of adipose tissue inflammation, antiinflammatory strategies including salicylate treatment and genetic suppression of myeloid NF-kappaB signaling that correct insulin resistance in obesity were ineffective in the lipodystrophic mice. We further showed that adipose tissue macrophages (ATMs) in lipodystrophy and obesity are very different in terms of activation state, gene expression patterns, and response to lipopolysaccharide. Although ATMs are even more abundant in lipodystrophy than in obesity, they have distinct phenotypes and likely roles in tissue remodeling, but do not appear to be involved in the pathogenesis of insulin resistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Transgenic Mice Overexpressing SREBP-1a in Male ob/ob Mice Exhibit Lipodystrophy and Exacerbate Insulin Resistance.Endocrinology. 2018 Jun 1;159(6):2308-2323. doi: 10.1210/en.2017-03179. Endocrinology. 2018. PMID: 29668871
-
FNDC5 attenuates adipose tissue inflammation and insulin resistance via AMPK-mediated macrophage polarization in obesity.Metabolism. 2018 Jun;83:31-41. doi: 10.1016/j.metabol.2018.01.013. Epub 2018 Jan 31. Metabolism. 2018. PMID: 29374559
-
A novel regulator of macrophage activation: miR-223 in obesity-associated adipose tissue inflammation.Circulation. 2012 Jun 12;125(23):2892-903. doi: 10.1161/CIRCULATIONAHA.111.087817. Epub 2012 May 11. Circulation. 2012. PMID: 22580331
-
Properties and functions of adipose tissue macrophages in obesity.Immunology. 2018 Dec;155(4):407-417. doi: 10.1111/imm.13002. Epub 2018 Oct 19. Immunology. 2018. PMID: 30229891 Free PMC article. Review.
-
Recent advances in the relationship between obesity, inflammation, and insulin resistance.Eur Cytokine Netw. 2006 Mar;17(1):4-12. Eur Cytokine Netw. 2006. PMID: 16613757 Review.
Cited by
-
Therapeutic value of brown adipose tissue: Correcting metabolic disease through generating healthy fat.Adipocyte. 2012 Oct 1;1(4):250-255. doi: 10.4161/adip.21042. Adipocyte. 2012. PMID: 23700541 Free PMC article.
-
Loss of BMP receptor type 1A in murine adipose tissue attenuates age-related onset of insulin resistance.Diabetologia. 2016 Aug;59(8):1769-77. doi: 10.1007/s00125-016-3990-8. Epub 2016 May 21. Diabetologia. 2016. PMID: 27209464 Free PMC article.
-
A Small Molecule, UAB126, Reverses Diet-Induced Obesity and its Associated Metabolic Disorders.Diabetes. 2020 Sep;69(9):2003-2016. doi: 10.2337/db19-1001. Epub 2020 Jul 1. Diabetes. 2020. PMID: 32611548 Free PMC article.
-
Systemic Dermatitis Model Mice Exhibit Atrophy of Visceral Adipose Tissue and Increase Stromal Cells via Skin-Derived Inflammatory Cytokines.Int J Mol Sci. 2020 May 9;21(9):3367. doi: 10.3390/ijms21093367. Int J Mol Sci. 2020. PMID: 32397568 Free PMC article.
-
Apoptosis, mastocytosis, and diminished adipocytokine gene expression accompany reduced epididymal fat mass in long-standing diet-induced obese mice.Lipids Health Dis. 2011 Nov 3;10:198. doi: 10.1186/1476-511X-10-198. Lipids Health Dis. 2011. PMID: 22051061 Free PMC article.
References
-
- Garg A. Acquired and inherited lipodystrophies. N Engl J Med. 2004;350:1220–1234. - PubMed
-
- Villarroya F, Domingo P, Giralt M. Lipodystrophy in HIV 1-infected patients: Lessons for obesity research. Int J Obes (Lond) 2007;31:1763–1776. - PubMed
-
- Heilbronn L, Smith SR, Ravussin E. Failure of fat cell proliferation, mitochondrial function and fat oxidation results in ectopic fat storage, insulin resistance and type II diabetes mellitus. Int J Obes Relat Metab Disord. 2004;28(Suppl 4):S12–S21. - PubMed
-
- Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: Defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest. 2002;32(Suppl 3):14–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

