The DM domain protein DMRT1 is a dose-sensitive regulator of fetal germ cell proliferation and pluripotency
- PMID: 20007774
- PMCID: PMC2799724
- DOI: 10.1073/pnas.0905431106
The DM domain protein DMRT1 is a dose-sensitive regulator of fetal germ cell proliferation and pluripotency
Abstract
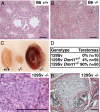
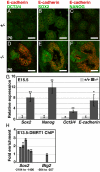
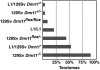
Dmrt1 (doublesex and mab-3 related transcription factor 1) is a conserved transcriptional regulator of male differentiation required for testicular development in vertebrates. Here, we show that in mice of the 129Sv strain, loss of Dmrt1 causes a high incidence of teratomas, whereas these tumors do not form in Dmrt1 mutant C57BL/6J mice. Conditional gene targeting indicates that Dmrt1 is required in fetal germ cells but not in Sertoli cells to prevent teratoma formation. Mutant 129Sv germ cells undergo apparently normal differentiation up to embryonic day 13.5 (E13.5), but some cells fail to arrest mitosis and ectopically express pluripotency markers. Expression analysis and chromatin immunoprecipitation identified DMRT1 target genes, whose missexpression may underlie teratoma formation. DMRT1 indirectly activates the GDNF coreceptor Ret, and it directly represses the pluripotency regulator Sox2. Analysis of human germ cell tumors reveals similar gene expression changes correlated to DMRT1 levels. Dmrt1 behaves genetically as a dose-sensitive tumor suppressor gene in 129Sv mice, and natural variation in Dmrt1 activity can confer teratoma susceptibility. This work reveals a genetic link between testicular dysgenesis, pluripotency regulation, and teratoma susceptibility that is highly sensitive to genetic background and to gene dosage.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Interaction between DMRT1 function and genetic background modulates signaling and pluripotency to control tumor susceptibility in the fetal germ line.Dev Biol. 2013 May 1;377(1):67-78. doi: 10.1016/j.ydbio.2013.02.014. Epub 2013 Mar 6. Dev Biol. 2013. PMID: 23473982 Free PMC article.
-
Testicular teratomas: an intersection of pluripotency, differentiation and cancer biology.Int J Dev Biol. 2013;57(2-4):201-10. doi: 10.1387/ijdb.130136bc. Int J Dev Biol. 2013. PMID: 23784831 Review.
-
Derivation of pluripotent stem cells from nascent undifferentiated teratoma.Dev Biol. 2019 Feb 1;446(1):43-55. doi: 10.1016/j.ydbio.2018.11.020. Epub 2018 Dec 5. Dev Biol. 2019. PMID: 30529251
-
Cell type-autonomous and non-autonomous requirements for Dmrt1 in postnatal testis differentiation.Dev Biol. 2007 Jul 15;307(2):314-27. doi: 10.1016/j.ydbio.2007.04.046. Epub 2007 May 3. Dev Biol. 2007. PMID: 17540358 Free PMC article.
-
Germ cell tumors of the gonads: a selective review emphasizing problems in differential diagnosis, newly appreciated, and controversial issues.Mod Pathol. 2005 Feb;18 Suppl 2:S61-79. doi: 10.1038/modpathol.3800310. Mod Pathol. 2005. PMID: 15761467 Review.
Cited by
-
Fetal radiation exposure induces testicular cancer in genetically susceptible mice.PLoS One. 2012;7(2):e32064. doi: 10.1371/journal.pone.0032064. Epub 2012 Feb 13. PLoS One. 2012. PMID: 22348147 Free PMC article.
-
A novel evolutionary conserved mechanism of RNA stability regulates synexpression of primordial germ cell-specific genes prior to the sex-determination stage in medaka.PLoS Biol. 2019 Apr 4;17(4):e3000185. doi: 10.1371/journal.pbio.3000185. eCollection 2019 Apr. PLoS Biol. 2019. PMID: 30947255 Free PMC article.
-
All (C57BL/6) Mice are not Created Equal.Front Neurosci. 2011 Feb 23;5:10. doi: 10.3389/fnins.2011.00010. eCollection 2011. Front Neurosci. 2011. PMID: 21390289 Free PMC article. No abstract available.
-
1700108J01Rik and 1700101O22Rik are mouse testis-specific long non-coding RNAs.Histochem Cell Biol. 2018 May;149(5):517-527. doi: 10.1007/s00418-018-1642-4. Epub 2018 Feb 6. Histochem Cell Biol. 2018. PMID: 29411102
-
Tumor loci and their interactions on mouse chromosome 19 that contribute to testicular germ cell tumors.BMC Genet. 2014 May 30;15:65. doi: 10.1186/1471-2156-15-65. BMC Genet. 2014. PMID: 24886204 Free PMC article.
References
-
- Guan K, et al. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature. 2006;440:1199–1203. - PubMed
-
- Matsui Y, Zsebo K, Hogan BL. Derivation of pluripotential embryonic stem cells from murine primordial germ cells in culture. Cell. 1992;70:841–847. - PubMed
-
- Kanatsu-Shinohara M, et al. Generation of pluripotent stem cells from neonatal mouse testis. Cell. 2004;119:1001–1012. - PubMed
-
- Labosky PA, Barlow DP, Hogan BL. Mouse embryonic germ (EG) cell lines: Transmission through the germline and differences in the methylation imprint of insulin-like growth factor 2 receptor (Igf2r) gene compared with embryonic stem (ES) cell lines. Development. 1994;120:3197–3204. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

