Two-component protein-engineered physical hydrogels for cell encapsulation
- PMID: 20007785
- PMCID: PMC2791665
- DOI: 10.1073/pnas.0904851106
Two-component protein-engineered physical hydrogels for cell encapsulation
Abstract
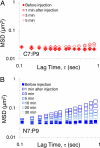
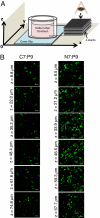
Current protocols to encapsulate cells within physical hydrogels require substantial changes in environmental conditions (pH, temperature, or ionic strength) to initiate gelation. These conditions can be detrimental to cells and are often difficult to reproduce, therefore complicating their use in clinical settings. We report the development of a two-component, molecular-recognition gelation strategy that enables cell encapsulation without environmental triggers. Instead, the two components, which contain multiple repeats of WW and proline-rich peptide domains, undergo a sol-gel phase transition upon simple mixing and hetero-assembly of the peptide domains. We term these materials mixing-induced, two-component hydrogels. Our results demonstrate use of the WW and proline-rich domains in protein-engineered materials and expand the library of peptides successfully designed into engineered proteins. Because both of these association domains are normally found intracellularly, their molecular recognition is not disrupted by the presence of additional biomolecules in the extracellular milieu, thereby enabling reproducible encapsulation of multiple cell types, including PC-12 neuronal-like cells, human umbilical vein endothelial cells, and murine adult neural stem cells. Precise variations in the molecular-level design of the two components including (i) the frequency of repeated association domains per chain and (ii) the association energy between domains enable tailoring of the hydrogel viscoelasticity to achieve plateau shear moduli ranging from approximately 9 to 50 Pa. Because of the transient physical crosslinks that form between association domains, these hydrogels are shear-thinning, injectable, and self-healing. Neural stem cells encapsulated in the hydrogels form stable three-dimensional cultures that continue to self-renew, differentiate, and sprout extended neurites.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Brandl F, Sommer F, Goepferich A. Rational design of hydrogels for tissue engineering: Impact of physical factors on cell behavior. Biomaterials. 2007;28:134–146. - PubMed
-
- Beaty CE, Saltzman WM. Controlled growth factor delivery induces differential neurite outgrowth in three-dimensional cell cultures. J Control Release. 1993;24:15–23.
-
- Kim S, Healy KE. Synthesis and characterization of injectable poly(N-isopropylacrylamide-co-acrylic acid) hydrogels with proteolytically degradable cross-links. Biomacromolecules. 2003;4:1214–1223. - PubMed
-
- Mahoney MJ, Anseth KS. Three-dimensional growth and function of neural tissue in degradable polyethylene glycol hydrogels. Biomaterials. 2006;27:2265–2274. - PubMed
-
- Petka WA, Harden JL, McGrath KP, Wirtz D, Tirrell DA. Reversible hydrogels from self-assembling artificial proteins. Science. 1998;281:389–392. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

