KRAS genotyping of paraffin-embedded colorectal cancer tissue in routine diagnostics: comparison of methods and impact of histology
- PMID: 20007841
- PMCID: PMC2797716
- DOI: 10.2353/jmoldx.2010.090079
KRAS genotyping of paraffin-embedded colorectal cancer tissue in routine diagnostics: comparison of methods and impact of histology
Abstract
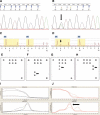
KRAS mutation testing before anti-epidermal growth factor receptor therapy of metastatic colorectal cancer has become mandatory in Europe. However, considerable uncertainty exists as to which methods for detection can be applied in a reproducible and economically sound manner in the routine diagnostic setting. To answer this question, we examined 263 consecutive routine paraffin slide specimens. Genomic DNA was extracted from microdissected tumor tissue. The DNA was analyzed prospectively by Sanger sequencing and array analysis as well as retrospectively by melting curve analysis and pyrosequencing; the results were correlated to tissue characteristics. The methods were then compared regarding the reported results, costs, and working times. Approximately 40% of specimens contained KRAS mutations, and the different methods reported concordant results (kappa values >0.9). Specimens harboring fewer than 10% tumor cells showed lower mutation rates regardless of the method used, and histoanatomical variables had no influence on the frequency of the mutations. Costs per assay were higher for array analysis and melting curve analysis when compared with the direct sequencing methods. However, for sequencing methods equipment costs were much higher. In conclusion, Sanger sequencing, array analysis, melting curve analysis, and pyrosequencing were equally effective for routine diagnostic KRAS mutation analysis; however, interpretation of mutation results in conjunction with histomorphologic tissue review and on slide tumor tissue dissection is required for accurate diagnosis.
Figures
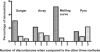
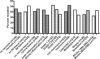
References
-
- Karim-Kos HE, de Vries E, Soerjomataram I, Lemmens V, Siesling S, Coebergh JW. Recent trends of cancer in Europe: a combined approach of incidence, survival and mortality for 17 cancer sites since the 1990s. Eur J Cancer. 2008;44:1345–1389. - PubMed
-
- Peeters M, Balfour J, Arnold D. Panitumumab—a fully human anti-EGFR monoclonal antibody for treatment of metastatic colorectal cancer. Aliment Pharmacol Ther. 2008;28:269–281. - PubMed
-
- Kuo T, Cho CD, Halsey J, Wakelee HA, Advani RH, Ford JM, Fisher GA, Sikic BI. Phase II study of gefitinib, fluorouracil, leucovorin, and oxaliplatin therapy in previously treated patients with metastatic colorectal cancer. J Clin Oncol. 2005;23:5613–5619. - PubMed
-
- Santoro A, Comandone A, Rimassa L, Granetti C, Lorusso V, Oliva C, Ronzoni M, Siena S, Zuradelli M, Mari E, Pressiani T, Carnaghi C. A phase II randomized multicenter trial of gefitinib plus FOLFIRI and FOLFIRI alone in patients with metastatic colorectal cancer. Ann Oncol. 2008;19:1888–1893. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

