Modeling complex workflow in molecular diagnostics: design specifications of laboratory software for support of personalized medicine
- PMID: 20007844
- PMCID: PMC2797718
- DOI: 10.2353/jmoldx.2010.090082
Modeling complex workflow in molecular diagnostics: design specifications of laboratory software for support of personalized medicine
Abstract
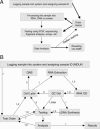
One of the hurdles to achieving personalized medicine has been implementing the laboratory processes for performing and reporting complex molecular tests. The rapidly changing test rosters and complex analysis platforms in molecular diagnostics have meant that many clinical laboratories still use labor-intensive manual processing and testing without the level of automation seen in high-volume chemistry and hematology testing. We provide here a discussion of design requirements and the results of implementation of a suite of lab management tools that incorporate the many elements required for use of molecular diagnostics in personalized medicine, particularly in cancer. These applications provide the functionality required for sample accessioning and tracking, material generation, and testing that are particular to the evolving needs of individualized molecular diagnostics. On implementation, the applications described here resulted in improvements in the turn-around time for reporting of more complex molecular test sets, and significant changes in the workflow. Therefore, careful mapping of workflow can permit design of software applications that simplify even the complex demands of specialized molecular testing. By incorporating design features for order review, software tools can permit a more personalized approach to sample handling and test selection without compromising efficiency.
Figures





References
-
- Lippi G, Guidi GC, Mattiuzzi C, Plebani M. Preanalytical variability: the dark side of the moon in laboratory testing. Clin Chem Lab Med. 2006;44:358–365. - PubMed
-
- Ohmann C, Kuchinke W. Future developments of medical informatics from the viewpoint of networked clinical research. Interoperability and integration. Methods Inf Med. 2009;48:45–54. - PubMed
-
- Burren OS, Healy BC, Lam AC, Schuilenburg H, Dolman GE, Everett VH, Laneri D, Nutland S, Rance HE, Payne F, Smyth D, Lowe C, Barratt BJ, Twells RC, Rainbow DB, Wicker LS, Todd JA, Walker NM, Smink LJ. Development of an integrated genome informatics, data management and workflow infrastructure: a toolbox for the study of complex disease genetics. Hum Genomics. 2004;1:98–109. - PMC - PubMed
-
- Yan Q. The integration of personalized and systems medicine: bioinformatics support for pharmacogenomics and drug discovery. Methods Mol Biol. 2008;448:1–19. - PubMed
-
- Amos J, Patnaik M. Commercial molecular diagnostics in the U.S.: The Human Genome Project to the clinical laboratory. Hum Mutat. 2002;19:324–333. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

