MicroRNA-206 delays ALS progression and promotes regeneration of neuromuscular synapses in mice
- PMID: 20007902
- PMCID: PMC2796560
- DOI: 10.1126/science.1181046
MicroRNA-206 delays ALS progression and promotes regeneration of neuromuscular synapses in mice
Abstract
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease characterized by loss of motor neurons, denervation of target muscles, muscle atrophy, and paralysis. Understanding ALS pathogenesis may require a fuller understanding of the bidirectional signaling between motor neurons and skeletal muscle fibers at neuromuscular synapses. Here, we show that a key regulator of this signaling is miR-206, a skeletal muscle-specific microRNA that is dramatically induced in a mouse model of ALS. Mice that are genetically deficient in miR-206 form normal neuromuscular synapses during development, but deficiency of miR-206 in the ALS mouse model accelerates disease progression. miR-206 is required for efficient regeneration of neuromuscular synapses after acute nerve injury, which probably accounts for its salutary effects in ALS. miR-206 mediates these effects at least in part through histone deacetylase 4 and fibroblast growth factor signaling pathways. Thus, miR-206 slows ALS progression by sensing motor neuron injury and promoting the compensatory regeneration of neuromuscular synapses.
Figures
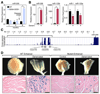
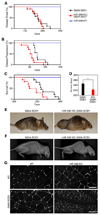
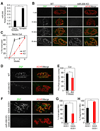
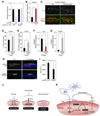
Comment in
-
Medicine. A reinnervating microRNA.Science. 2009 Dec 11;326(5959):1494-5. doi: 10.1126/science.1183842. Science. 2009. PMID: 20007892 No abstract available.
-
Commentary [Research highlights (To miR or not to miR: that is the question in ALS disease)].CNS Neurol Disord Drug Targets. 2011 Sep 1;10(6):648-9. doi: 10.2174/187152711797247812. CNS Neurol Disord Drug Targets. 2011. PMID: 21838675 No abstract available.
Similar articles
-
Muscle histone deacetylase 4 upregulation in amyotrophic lateral sclerosis: potential role in reinnervation ability and disease progression.Brain. 2013 Aug;136(Pt 8):2359-68. doi: 10.1093/brain/awt164. Epub 2013 Jul 3. Brain. 2013. PMID: 23824486
-
Medicine. A reinnervating microRNA.Science. 2009 Dec 11;326(5959):1494-5. doi: 10.1126/science.1183842. Science. 2009. PMID: 20007892 No abstract available.
-
Histone deacetylase 4 protects from denervation and skeletal muscle atrophy in a murine model of amyotrophic lateral sclerosis.EBioMedicine. 2019 Feb;40:717-732. doi: 10.1016/j.ebiom.2019.01.038. Epub 2019 Feb 1. EBioMedicine. 2019. PMID: 30713114 Free PMC article.
-
Neuromuscular Junction Dismantling in Amyotrophic Lateral Sclerosis.Int J Mol Sci. 2017 Oct 3;18(10):2092. doi: 10.3390/ijms18102092. Int J Mol Sci. 2017. PMID: 28972545 Free PMC article. Review.
-
Therapeutics Targeting Skeletal Muscle in Amyotrophic Lateral Sclerosis.Biomolecules. 2024 Jul 22;14(7):878. doi: 10.3390/biom14070878. Biomolecules. 2024. PMID: 39062592 Free PMC article. Review.
Cited by
-
Improved knee extensor strength with resistance training associates with muscle specific miRNAs in older adults.Exp Gerontol. 2015 Feb;62:7-13. doi: 10.1016/j.exger.2014.12.014. Epub 2015 Jan 2. Exp Gerontol. 2015. PMID: 25560803 Free PMC article.
-
HDAC4 as a potential therapeutic target in neurodegenerative diseases: a summary of recent achievements.Front Cell Neurosci. 2015 Feb 24;9:42. doi: 10.3389/fncel.2015.00042. eCollection 2015. Front Cell Neurosci. 2015. PMID: 25759639 Free PMC article. Review.
-
MicroRNAs in Neurotoxicity.J Toxicol. 2012;2012:870150. doi: 10.1155/2012/870150. Epub 2012 Mar 6. J Toxicol. 2012. PMID: 22523492 Free PMC article.
-
Regulation of miRNAs in human skeletal muscle following acute endurance exercise and short-term endurance training.J Physiol. 2013 Sep 15;591(18):4637-53. doi: 10.1113/jphysiol.2013.255695. Epub 2013 Jun 24. J Physiol. 2013. PMID: 23798494 Free PMC article.
-
MicroRNAs and Long Non-coding RNAs in Genetic Diseases.Mol Diagn Ther. 2019 Apr;23(2):155-171. doi: 10.1007/s40291-018-0380-6. Mol Diagn Ther. 2019. PMID: 30610665 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

