Liver X receptor agonists augment human islet function through activation of anaplerotic pathways and glycerolipid/free fatty acid cycling
- PMID: 20007976
- PMCID: PMC2820768
- DOI: 10.1074/jbc.M109.064659
Liver X receptor agonists augment human islet function through activation of anaplerotic pathways and glycerolipid/free fatty acid cycling
Abstract
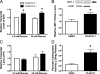
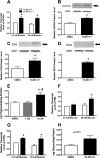
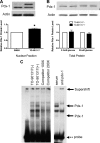
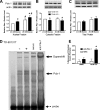
Recent studies in rodent models suggest that liver X receptors (LXRs) may play an important role in the maintenance of glucose homeostasis and islet function. To date, however, no studies have comprehensively examined the role of LXRs in human islet biology. Human islets were isolated from non-diabetic donors and incubated in the presence or absence of two synthetic LXR agonists, TO-901317 and GW3965, under conditions of low and high glucose. LXR agonist treatment enhanced both basal and stimulated insulin secretion, which corresponded to an increase in the expression of genes involved in anaplerosis and reverse cholesterol transport. Furthermore, enzyme activity of pyruvate carboxylase, a key regulator of pyruvate cycling and anaplerotic flux, was also increased. Whereas LXR agonist treatment up-regulated known downstream targets involved in lipogenesis, we observed no increase in the accumulation of intra-islet triglyceride at the dose of agonist used in our study. Moreover, LXR activation increased expression of the genes encoding hormone-sensitive lipase and adipose triglyceride lipase, two enzymes involved in lipolysis and glycerolipid/free fatty acid cycling. Chronically, insulin gene expression was increased after treatment with TO-901317, and this was accompanied by increased Pdx-1 nuclear protein levels and enhanced Pdx-1 binding to the insulin promoter. In conclusion, our data suggest that LXR agonists have a direct effect on the islet to augment insulin secretion and expression, actions that should be considered either as therapeutic or unintended side effects, as these agents are developed for clinical use.
Figures
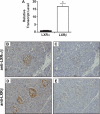
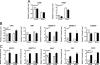
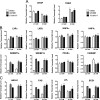
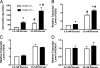
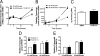
References
-
- Edwards P. A., Kennedy M. A., Mak P. A. (2002) Vascul. Pharmacol. 38, 249–256 - PubMed
-
- Varga G., Su C. (2007) BioDrugs 21, 117–124 - PubMed
-
- Joseph S. B., Castrillo A., Laffitte B. A., Mangelsdorf D. J., Tontonoz P. (2003) Nat. Med. 9, 213–219 - PubMed
-
- Scholz H., Lund T., Dahle M. K., Collins J. L., Korsgren O., Wang J. E., Foss A. (2009) Diabetologia 52, 1352–1362 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

