Partial agonist activity of the progesterone receptor antagonist RU486 mediated by an amino-terminal domain coactivator and phosphorylation of serine400
- PMID: 20008003
- PMCID: PMC2817605
- DOI: 10.1210/me.2008-0081
Partial agonist activity of the progesterone receptor antagonist RU486 mediated by an amino-terminal domain coactivator and phosphorylation of serine400
Abstract
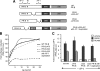
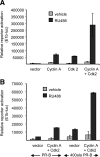
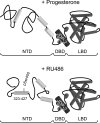
Jun dimerization protein-2 (JDP-2) is a progesterone receptor (PR) coregulatory protein that acts by inducing structure and transcriptional activity in the disordered amino-terminal domain (NTD) of PR. JDP-2 can also potentiate the partial agonist activity of the PR antagonist RU486 by mechanisms that have not been defined. Functional mutagenesis experiments revealed that a subregion of the NTD (amino acids 323-427) was required for the partial agonist activity of RU486 induced by PR interaction with JDP-2. However, this subregion was not required for JDP-2 enhancement of the activity of progestin agonists. Mutation of phosphorylation sites within this region of the NTD showed that phosphorylation of serine 400 was required for the partial agonist activity of RU486 stimulated by JDP-2, but was not required for activity of hormone agonist, either in the presence or absence of JDP-2. Cyclin-dependent kinase 2 (Cdk2)/cyclin A is a novel PR coregulator that binds the NTD and acts by phosphorylating steroid receptor coactivator-1 and modulating steroid receptor coactivator-1 interaction with PR. Cdk2/cyclin A also potentiated the partial agonist activity of RU486; however, phosphorylation of serine 400 was not required, indicating that JDP-2 and Cdk2/cyclin A act by distinct mechanisms. We conclude that PR bound to RU486 and associated with JDP-2 adopts an active conformation in a subregion of the NTD requiring phosphorylation of serine 400 that is distinct from that promoted by progestin agonists. These data underscore the structural flexibility of the NTD of PR, and the ability of steroid ligands together with interacting proteins to affect the conformation and activity of the NTD.
Figures



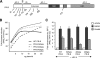
Similar articles
-
Jun dimerization protein 2 functions as a progesterone receptor N-terminal domain coactivator.Mol Cell Biol. 2002 Aug;22(15):5451-66. doi: 10.1128/MCB.22.15.5451-5466.2002. Mol Cell Biol. 2002. PMID: 12101239 Free PMC article.
-
Agonist and antagonists induce homodimerization and mixed ligand heterodimerization of human progesterone receptors in vivo by a mammalian two-hybrid assay.Mol Endocrinol. 1998 Dec;12(12):1914-30. doi: 10.1210/mend.12.12.0210. Mol Endocrinol. 1998. PMID: 9849965
-
Hormone-dependent interaction between the amino- and carboxyl-terminal domains of progesterone receptor in vitro and in vivo.Mol Endocrinol. 1999 Jun;13(6):910-24. doi: 10.1210/mend.13.6.0300. Mol Endocrinol. 1999. PMID: 10379890
-
Mechanisms controlling agonist and antagonist potential of selective progesterone receptor modulators (SPRMs).Semin Reprod Med. 2005 Feb;23(1):9-21. doi: 10.1055/s-2005-864030. Semin Reprod Med. 2005. PMID: 15714386 Review.
-
Cyclin dependent kinase 2 and the regulation of human progesterone receptor activity.Steroids. 2007 Feb;72(2):202-9. doi: 10.1016/j.steroids.2006.11.025. Epub 2007 Jan 4. Steroids. 2007. PMID: 17207508 Free PMC article. Review.
Cited by
-
Allosteric modulators of steroid hormone receptors: structural dynamics and gene regulation.Endocr Rev. 2012 Apr;33(2):271-99. doi: 10.1210/er.2011-1033. Epub 2012 Mar 20. Endocr Rev. 2012. PMID: 22433123 Free PMC article. Review.
-
p38 and p42/44 MAPKs differentially regulate progesterone receptor A and B isoform stabilization.Mol Endocrinol. 2011 Oct;25(10):1710-24. doi: 10.1210/me.2011-1042. Epub 2011 Aug 4. Mol Endocrinol. 2011. PMID: 21816898 Free PMC article.
-
Selective progesterone receptor modulators (SPRMs): progesterone receptor action, mode of action on the endometrium and treatment options in gynecological therapies.Expert Opin Ther Targets. 2016 Sep;20(9):1045-54. doi: 10.1080/14728222.2016.1180368. Epub 2016 May 14. Expert Opin Ther Targets. 2016. PMID: 27138351 Free PMC article. Review.
-
Membrane Progesterone Receptors (mPRs/PAQRs) Are Going beyond Its Initial Definitions.Membranes (Basel). 2023 Feb 22;13(3):260. doi: 10.3390/membranes13030260. Membranes (Basel). 2023. PMID: 36984647 Free PMC article. Review.
-
Structural proteomics defines a sequential priming mechanism for the progesterone receptor.Nat Commun. 2025 May 12;16(1):4403. doi: 10.1038/s41467-025-59458-y. Nat Commun. 2025. PMID: 40355435 Free PMC article.
References
-
- Li X, O'Malley BW 2003 Unfolding the action of progesterone receptors. J Biol Chem 278:39261–39264 - PubMed
-
- Tora L, White J, Brou C, Tasset D, Webster N, Scheer E, Chambon P 1989 The human estrogen receptor has two independent non-acidic transcriptional activation functions. Cell 59:477–487 - PubMed
-
- Meyer ME, Quirin-Stricker C, Lerouge T, Bocquel MT, Gronemeyer H 1992 A limiting factor mediates the differential activation of promoters by the human progesterone receptor isoforms. J Biol Chem 267:10882–10887 - PubMed
-
- Giangrande PH, McDonnel DP 1999 The A and B isoforms of the human progesterone receptor: two functionally different transcription factors encoded by a single gene. Recent Prog Horm Res 45:291–313 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

